High affinity monoclonal antibodies targeting glypican-1 and methods of use
A technology of monoclonal antibody and phosphatidylinositol, applied in proteoglycans, chemical instruments and methods, polypeptides containing positioning/targeting motifs, etc., can solve the elusive curative effect of solid tumors and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0320] Example 1: Isolation and Characterization of GPC1-Specific Monoclonal Antibodies
[0321] This example describes two GPC1-specific monoclonal antibodies isolated from phage display libraries and mouse hybridomas. Mouse monoclonal antibody HM2 (SEQ ID NO:2 and SEQ ID NO:4) binds to the C-lobe of GPC1 near the cell surface, camel single domain antibody D4 (SEQ ID NO:6) recognizes the GPC1 protein core conformational epitope.
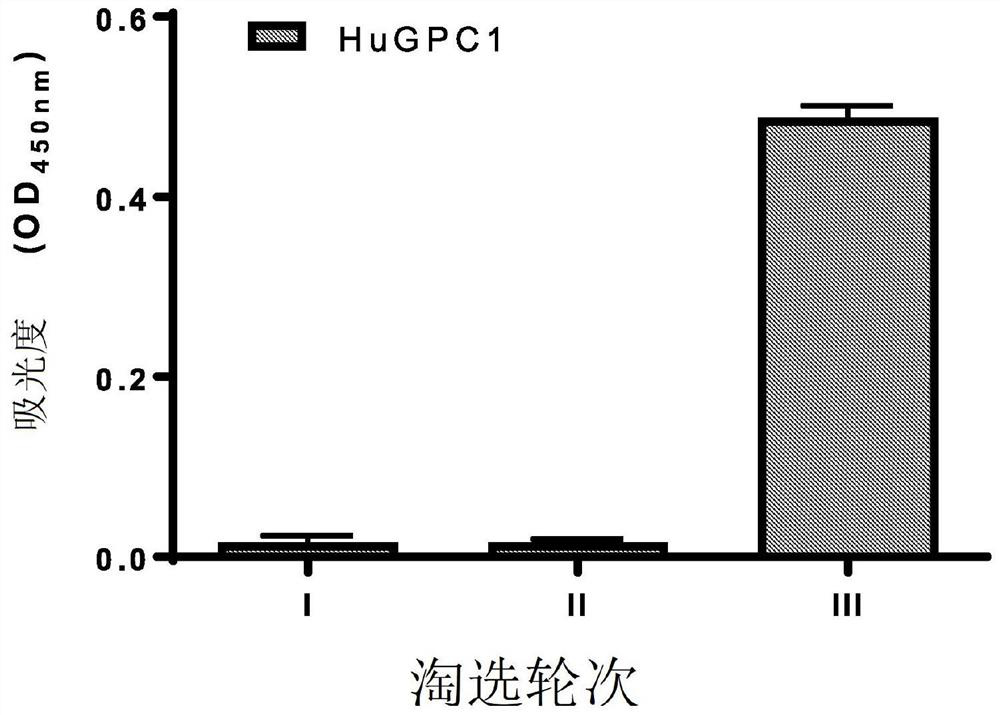
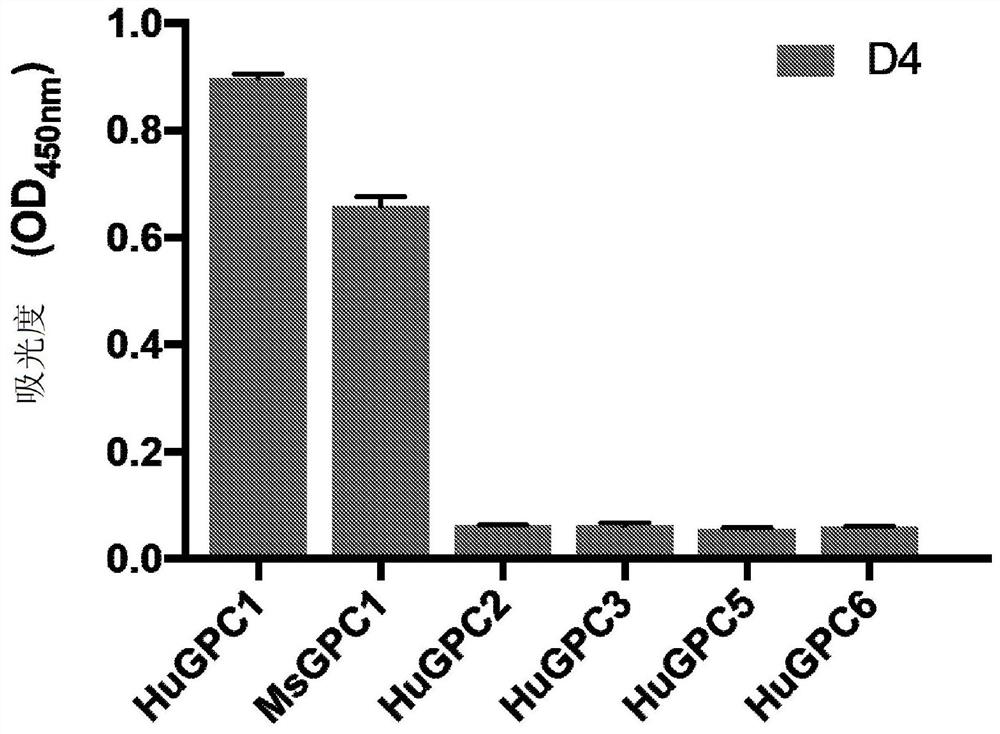
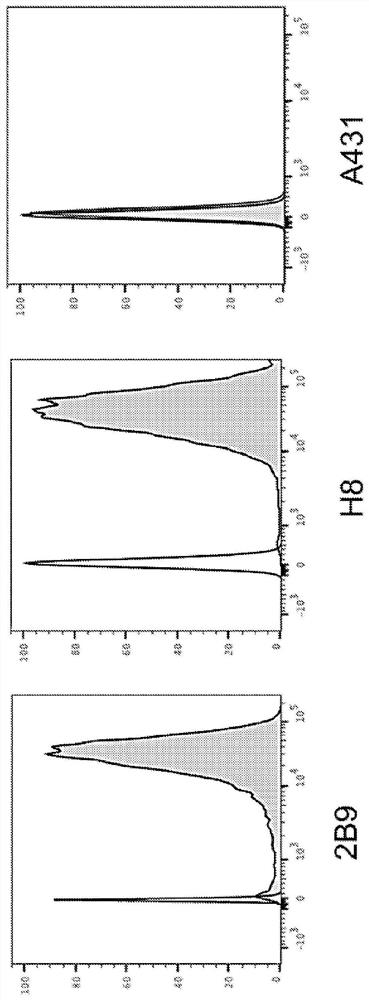
[0322] After three rounds of panning, antibody D4 ( Figure 1A ). Monoclonal phage ELISA analysis of D4 demonstrated that D4 specifically bound human and mouse GPC1, but did not exhibit any significant binding to human GPC2, GPC3, GPC5, or GPC6 ( Figure 1B ). Binding of D4 to GPC1-expressing tumor cells was assessed by flow cytometry. D4 exhibited significant binding to GPC1-overexpressing 2B9 KLM pancreatic cancer cells and GPC1-overexpressing H8 epidermoid cancer cells, but not to GPC1-negative A431 cells ( figure 2 ). use Kinetic analy...
Embodiment 2
[0329] Example 2: Immunotoxins based on D4 and HM2 antibodies
[0330] This example describes the generation and testing of immunotoxins consisting of D4 or HM2 antibodies and a truncated form of Pseudomonas exotoxin A lacking domain II (referred to herein as "LR").
[0331] immunotoxin sequence
[0332] Four different immunotoxins were generated: D4-LR, HM2-LR, D4-AAA-D4-LR and D4-GGS-D4-LR. The latter two immunotoxins consist of D4 dimers separated by a three amino acid linker (AAA or GGS). The nucleotide and amino acid sequences of the four immunotoxins are provided below. The LR coding sequence (for nucleotide sequences) and the LR portion of the amino acid sequence are underlined.
[0333] D4-LR nucleotide sequence (SEQ ID NO: 14)
[0334] ATGCAGGTGCAGCTGGTGGAGTCTGGGGGAGGCTTGGTGCAGCCCGGGGGGTCTCTGAGACTCTCCTGTGTAGCCTCTGGATACAGCTACAGTATTGGTTACATGGCCTGGTTCCGCCAGGCCCCAGGAAAGGAGCGCGCGTGGGTCGCGTCTCGATATACTGGTGACGGTGGCGCAGTCTTTGACGACGCCGTGAAGGGCCGATTCACCACCTCCCAAGAGAGTGCCGGGA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com