Systemic allergic response risk assessment in peanut oral immunotherapy
An immunotherapy and allergic reaction technology, applied in allergic diseases, biological testing, biological material analysis, etc., can solve problems such as difficult to explain the undeclared ingredients of food labels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
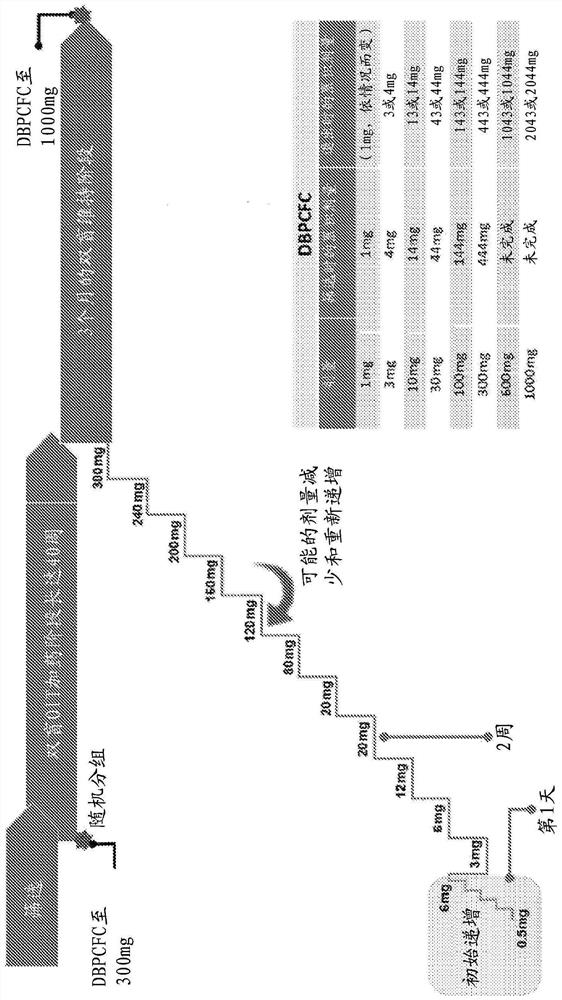
[0095] Example 1: European Phase 3 Clinical Trial Measuring Oral Immunotherapy Success of AR101 in Peanut Allergic Children test
[0096] The following study is a European multicenter randomized double-blind placebo-controlled clinical trial entitled "European AR101 Trial Measuring the Success of Oral Immunotherapy in Children with Peanut Allergy (ARTEMIS)", investigating the effect of a peanut protein preparation (AR101) on peanut allergy Efficacy and safety in an individual's characterized desensitized oral immunotherapy regimen. Pediatric and adolescent patients aged 4-17 years are considered eligible. All participants had a clinical history of peanut allergy (confirmed by screening a double-blind placebo-controlled food challenge (DBPCFC)) and a serum peanut-specific IgE (psIgE) ≥0.35 kU A / L (via UniCAP TM (Phadia AB, Uppsala, Sweden) confirmed within the past 12 months) and / or peanut skin prick test mean wheal diameter ≥3mm larger than negative control (eg, saline)...
Embodiment 2
[0119] The clinical safety test of embodiment 2.AR101
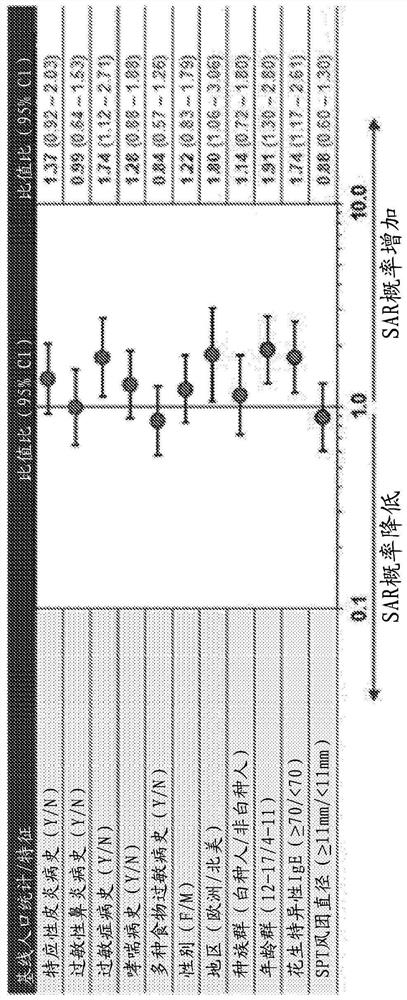
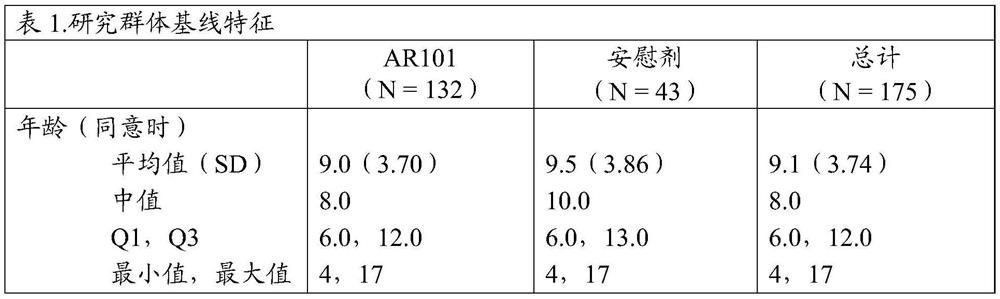
[0120] In addition to the studies discussed in Example 1, the efficacy and safety of a peanut protein formulation (AR101 ) in a characterized desensitization oral immunotherapy regimen for peanut-allergic individuals was investigated in the following four clinical trials. Two studies, called ARC003 and ARC007, were completed, and their open-label extension studies, called ARC004 and ARC011, are ongoing. For ongoing studies, the data presented are from the cut-off date of 15 December 2018.
[0121] ARC003 (also known as PALISADE) was a large double-blind placebo-controlled Phase 3 study of AR101 in peanut allergy patients aged 4 to 55 years and was the first to include a double-blind placebo at both entry and exit Controlled food challenge (DBPCFC) test. See Jones et al., "Efficacy and Safety of AR101 in PeanutAllergy: Results from a Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial (PALISADE)," J. Allergy Cl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com