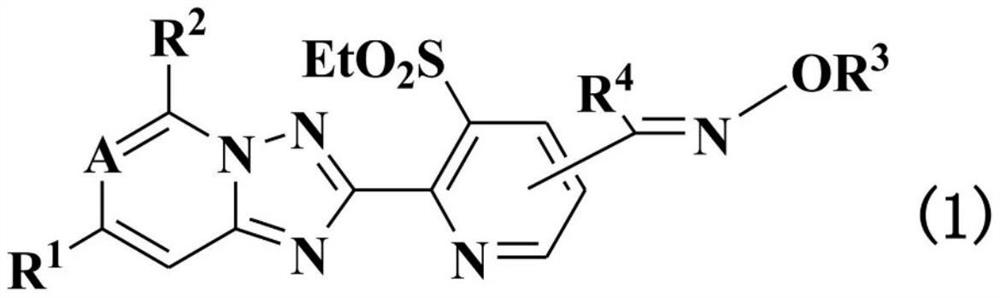
Condensed heterocyclic compound or salt thereof comprising nitrogen atom in cross-link, and agricultural pesticide containing said compound and method for using same
A technology of heterocyclic compounds and nitrogen atoms, applied in gardening methods, insecticides, nematicides, etc., can solve problems that have not been specifically disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
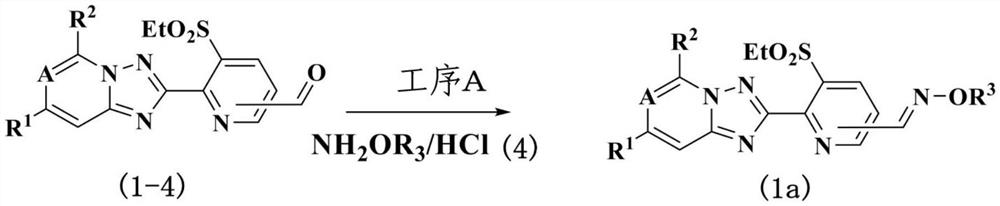
[0062] Preparation method of step [a]
[0063] The compound represented by the formula (1a) can be, and the compound represented by the formula (1-4) prepared in the preparation method 3 described later in the preparation method 3 is prepared in the presence of a base and an inert solvent. 4) The compound represented (NH) 2 Organ 3 Reaction and prepared.
[0064] As the base which can be used in this reaction, for example, an inorganic base such as sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium hydrogencarbonate can be mentioned; acetate such as potassium acetate; triethylamine , Diisopropylamine, 1,8-diazepine diprocyte [5.4.0] eclipanoamine such as 11 carbon-7-ene; pyridine, 4-dimethylaminopyridine and other nitrogen-containing aromatic compounds, etc. For its amount, it is usually used in the range of 1 x mole to 10 dimension relative to the compound represented by the formula (4).
[0065] As the inert solvent used i...
reference example 1
[0249] Reference Example 1. Preparation method of trifrofluoromethylpyrimidine-1-lysonium-1,6-diamine 2,4,6-trimethylsulfonate
[0250] [Chemical Formula 9]
[0251]
[0252]2,2,2-trifluoroacetic acid (4.4 g, 2.54 mmol, 2.9 mL) was added to a microwave tube having a magnetic stirrer, and then added (tert-butoxycarbonyl amino) 2, 4, 6- at 0 ° C. Trimethylbenzenesulfonate (1 g, 2.54 mmol). The reaction mixture was stirred at 0 ° C for 2 hours, and ice water was added, and the precipitate was recovered by filtration. Wash the moist filter cake with water, and dissolved in dichloromethane (5 mL), dried to sodium sulfate. The resulting solution was added dropwise to 6-trifluoromethylpyrimidine-4-amine (0.373 g, dichloromethane (5ml) (5ml) (5 ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5 ml) (5 ml) (5 ml) (5 ml) (5 ml) (5 ml) (5 ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5ml) (5ml). )middle. After 1 hour after this temperature and after one night at room...
reference example 2
[0253] Reference Example 2.2- (3-ethylsulfonyl-5-bromo-2-pyridyl) -7-trifluoromethyl-[1,2,4] triazole [1,5-C] pyrimidine preparation method
[0254] [Chemical Formula 10]
[0255]
[0256] 4-trifluoroamine-1-lyson-1,6-diamine 2,4,6-trimethylsulfonate (0.3 g, 0.791 mmol), 3-ethylsulfonyl-5-bromine Pyridin-2-carboxylic acid (0.284 g, 1.18 mmol) and 3- (ethylene aminomethylene) -N, N-dimethyl-propane-1-amine hydrochloride (0.18 g, 0.94 mmol) Dissolved in pyridine (2 mL), heated at 120 ° C for 3 hours. Then, the reaction mixture was injected into water, and the aqueous layer was extracted 3 times using EtOAc (ethyl acetate). Wash the combined organic layers in times of water and brine, use anhydrous NA 2 SO 4 (Sodium sulfate) was dried and filtered and concentrated under reduced pressure. The crude product was washed with diethyl ether and filtered to give a target product as a white powder (90 mg, 33%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com