Bacterial interception test device and test method
A test device and bacterial technology, which is applied in the field of bacterial retention test device, can solve the problems of increasing filtration resistance, affecting the verification result of filtration process, failing to meet the filtration pressure difference and flow rate at the same time, and achieving the effect of accurate filtration volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
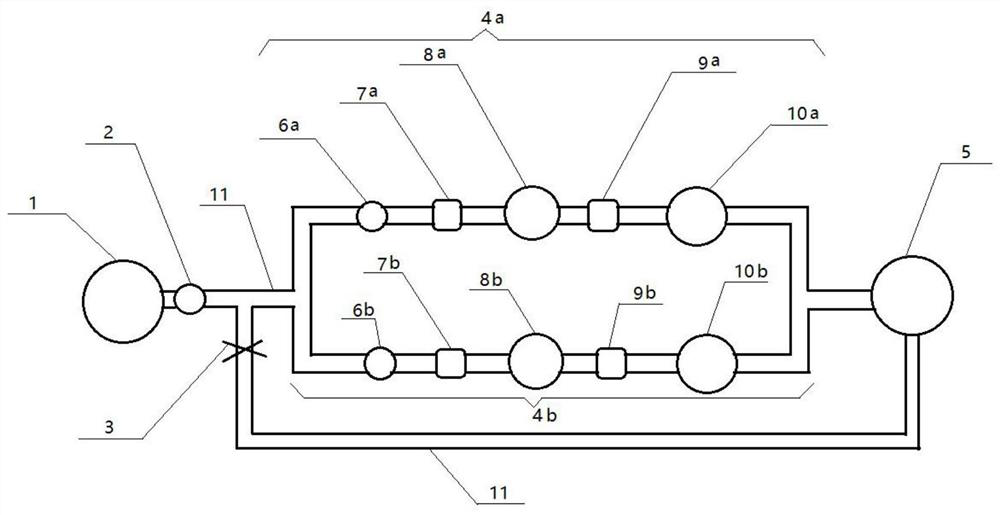
[0034] A bacterial retention test device, such as figure 1 Shown: including raw liquid tank 1, first peristaltic pump 2, valve 3, filtration passage 4 and circulation tank 5; said raw liquid tank 1, first peristaltic pump 2, filtration passage 4, circulation tank 5 pass through the The roads are connected sequentially, and the bottom of the circulation tank 5 is connected between the first peristaltic pump 2 and the filtration passage 4 through a pipeline 11 to form a communication loop to realize circulation filtration, and a valve 3 is set on the pipeline 11; the filtration passage 4 Including a positive control group and a verification group, the positive control group and the verification group are connected in parallel, and the second peristaltic pump 6, the first pressure gauge 7, the filter 8, the second pressure gauge 9 and the filter are connected in series in sequence on each group of passages. Membrane 10.
[0035] The filtering path 4 of the device includes a posi...
Embodiment 2
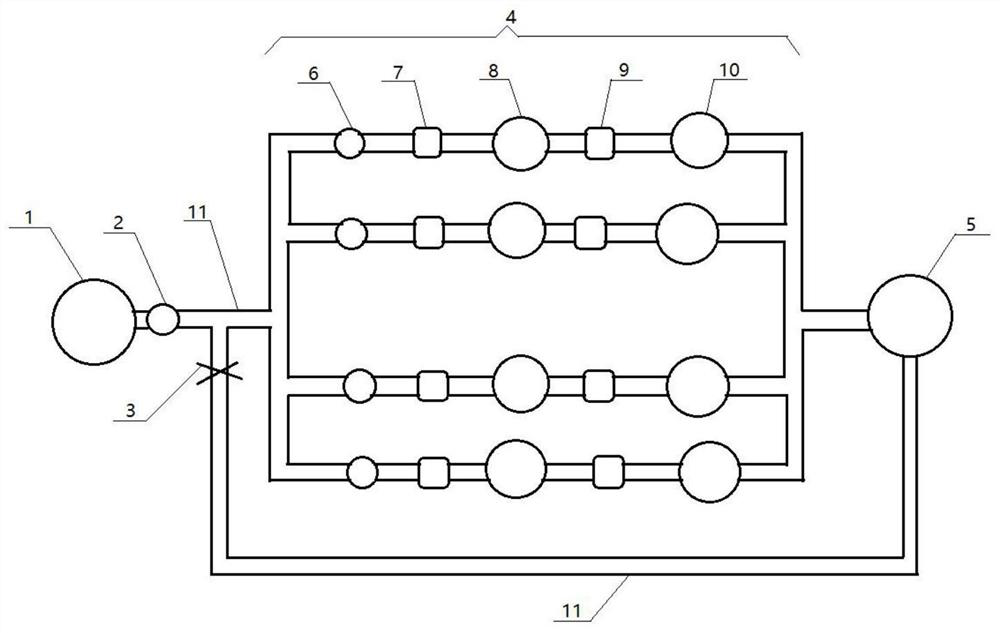
[0048] A bacterial retention test device, such as figure 2 Shown: including raw liquid tank 1, first peristaltic pump 2, valve 3, filtration passage 4 and circulation tank 5; said raw liquid tank 1, first peristaltic pump 2, filtration passage 4, circulation tank 5 pass through the The roads are connected sequentially, and the bottom of the circulation tank 5 is connected between the first peristaltic pump 2 and the filtration passage 4 through a pipeline 11 to form a communication loop to realize circulation filtration, and a valve 3 is set on the pipeline 11; the filtration passage 4 Including a positive control group and a verification group, the positive control group and the verification group are connected in parallel, and the second peristaltic pump 6, the first pressure gauge 7, the filter 8, the second pressure gauge 9 and the filter are connected in series in sequence on each group of passages. Membrane 10.
[0049] The filter path of the device includes a positive...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com