Chromene-based compounds, methods and uses thereof
A technology of compounds and hydrates, applied in organic chemistry, pharmaceutical formulations, drug combinations, etc., can solve problems such as impermissible molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0097] The present invention relates to a new class of benzopyranyl anticancer compounds. The present invention describes the anticancer properties of a new class of benzopyranyl anticancer compounds. The present invention also describes methods for the synthesis and isolation of new classes of benzopyranyl anticancer compounds.
[0098] In particular, the present invention describes methods for the synthesis of novel benzopyranyl compounds by combining at least two different molecules, thus generating compounds with relevant biological profiles of interest.
[0099] In one embodiment, 19 different benzopyran-imidazolyl compounds were isolated. Table 1 shows the benzopyranyl skeleton structure and 19 synthesized and isolated benzopyran-imidazolyl compounds.
[0100] Table 1: Structures of Benzopyranyl Compounds
[0101]
[0102]
[0103]
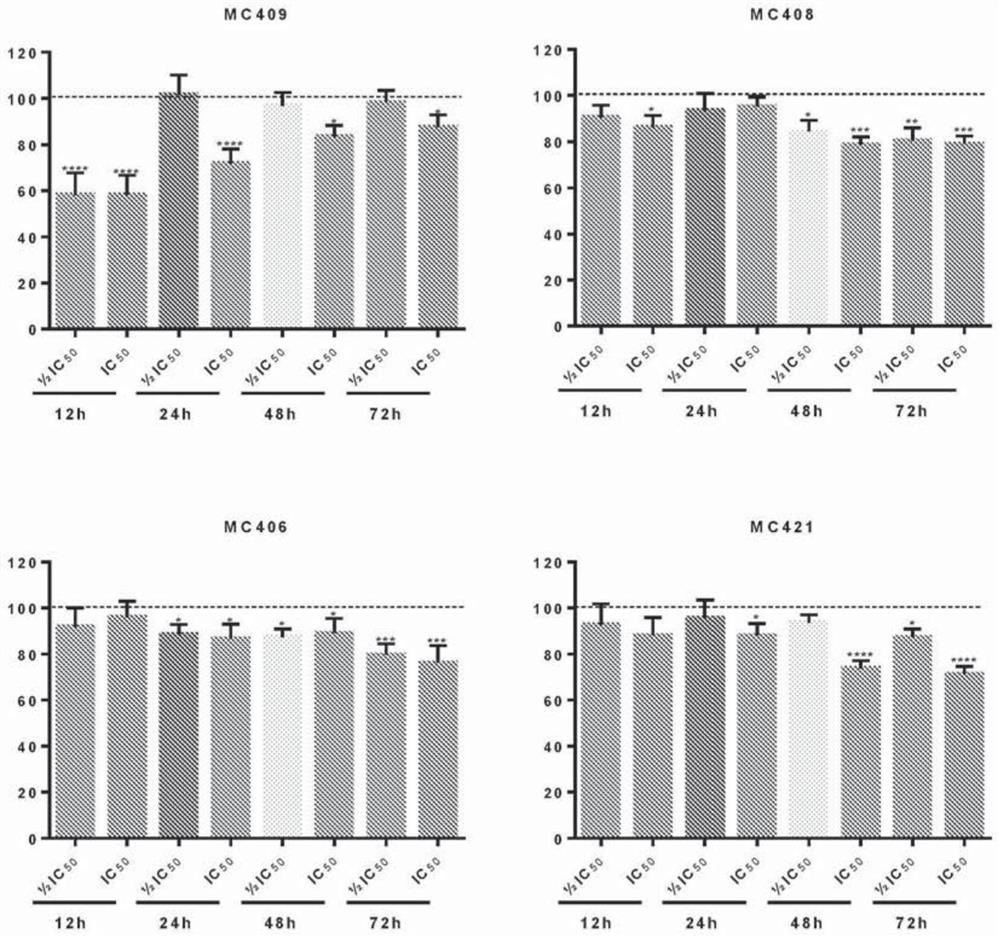
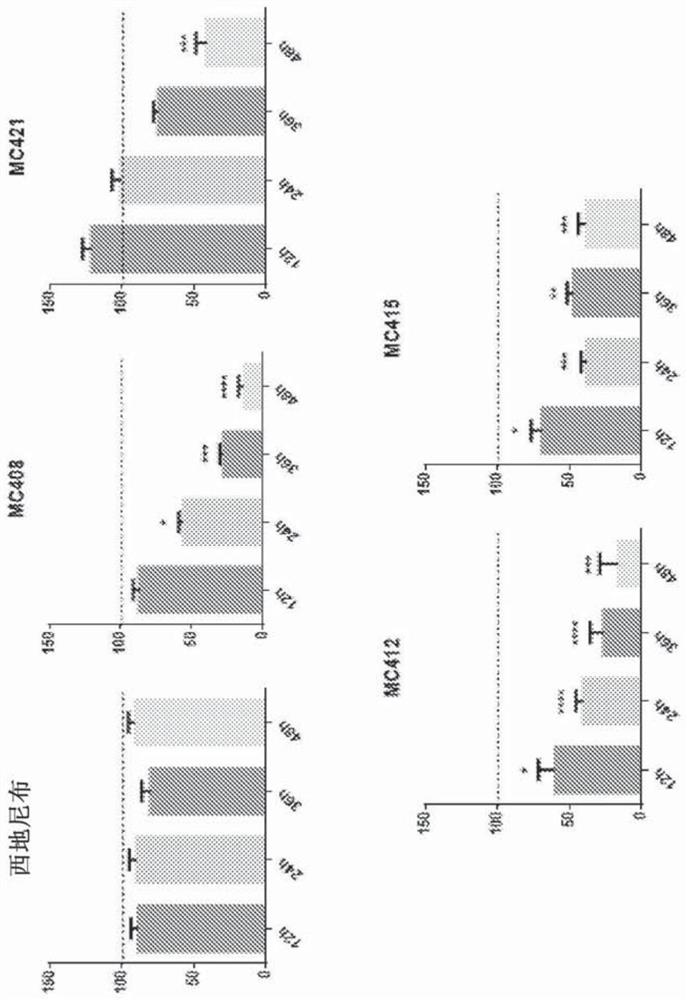
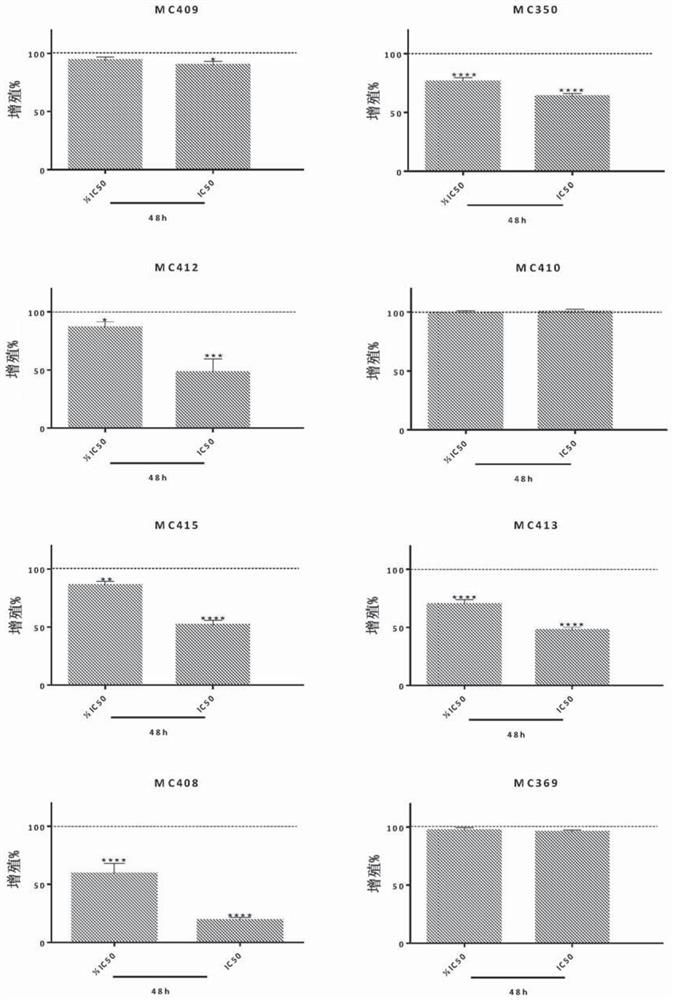
[0104] In one embodiment, the cell growth inhibition and IC of the synthesized and isolated benzopyranyl compounds are determin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com