Designed ankyrin repeat domains with improved stability
A repeat domain, ankyrin technology, applied in the field of disease treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1: Construction of designer anchors with binding specificity for serum albumin and improved stability protein repeat domain
[0136]Unexpectedly, it was observed that a protein consisting of the amino acid sequence corresponding to SEQ ID NO: 2 (originally described in WO2016156596) appeared to be unstable after incubation at elevated temperatures (see Examples 2 to 6), despite the fact that it is a variant with improved stability compared to an earlier designed ankyrin repeat domain (WO2012 / 069654) with binding specificity for serum albumin. It is therefore an object of the present invention to provide variants of SEQ ID NO: 2 which exhibit improved stability after incubation at elevated temperatures while maintaining serum albumin binding and pharmacokinetic properties. Through a highly iterative process involving several rounds of changing amino acids at multiple positions (e.g., comparable to the alanine scanning process well known to those skilled in th...
Embodiment 2
[0138] Example 2: Expression and purification of proteins
[0139] Proteins consisting of SEQ ID NOs: 2 to 6, additionally having a His-tag SEQ ID NO: 1 fused to its N-terminus, and consisting of SEQ ID NOs: 7 to 12, additionally having a His-tag fused to its N-terminus were produced in E. coli The terminal His-tagged protein of SEQ ID NO: 1 was purified to homogeneity and stored in PBS buffer. For clarity, proteins #2 to #6 are designed ankyrin repeat domains with binding specificity for serum albumin and proteins #7 to #12 are designed anchor protein domains containing binding specificity for serum albumin Recombinant binding proteins of protein repeat domains. Proteins #7 and #8 contain twice as much SEQ ID NO:2. Proteins #9 and #10 contain twice as much SEQ ID NO:3. Proteins #11 and #12 contain twice as much SEQ ID NO:4. Protein #7 of SEQ ID NO: 134 from WO2016156596 is well known to those skilled in the art, and protein #8 of SEQ ID NO: 21 from WO2018054971 is well ...
Embodiment 3
[0141] Example 3: Storage Stability Incubation
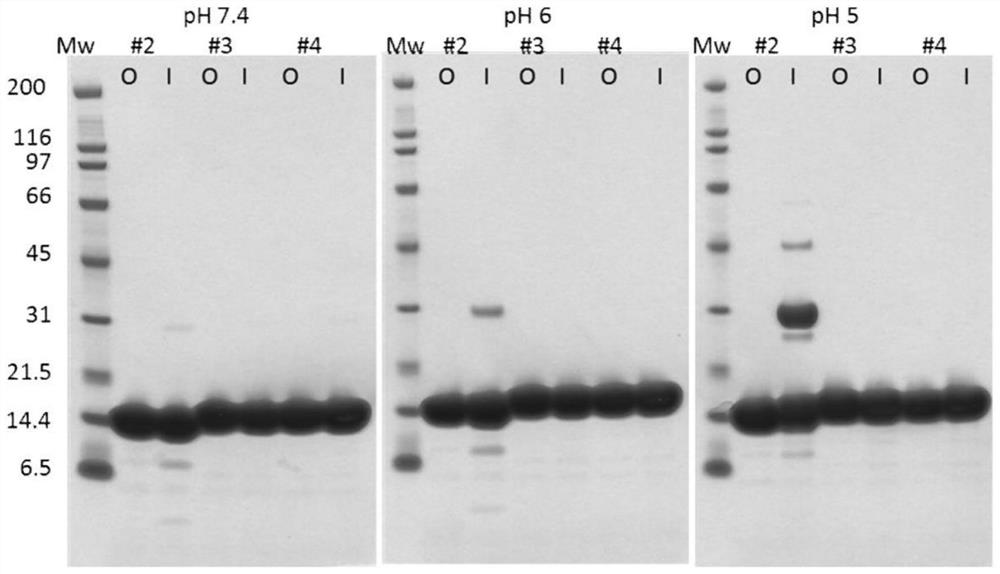
[0142] The protein of Example 2 was tested for 1 week (7 days) storage stability at various pH at 100 micromolar protein concentration and 60°C. Buffers used were PBS (pH 7.4; 137 mM NaCl, 10 mM phosphate and 2.7 mM KCl) or phosphate / citrate (pH 5.7; 375 mM Na2HPO4*2H2O and 150 mM NaCl, pH adjusted using 1M citrate monohydrate ), or phosphate / citrate (pH 4.75; 30 mM citric acid and 30 mM NaH2PO4, pH adjusted with sodium hydroxide), or phosphate / citrate / borate (pH 8.5, 30 mM citric acid, 30 mM NaH2PO4 , 30mM boric acid). When the protein is mixed with the corresponding buffer, the resulting pH values are pH 7.4, pH 6, pH 5 or pH 8.5, respectively. In parallel to the incubation at 60°C, an aliquot of the protein was incubated at -80°C for 1 week (7 days) as a control.
[0143] Stability during incubation at 60°C and at different pH is industrially relevant for the manufacture of designer ankyrin repeat domains or recombinan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com