Preparation method of haloxyfop-R-methyl
A high-efficiency technology of haloxyfop-pyl and trifluoromethylpyridine, which is applied in the direction of organic chemistry, can solve the problems of difficult preparation, complicated route, poor safety and environmental protection, etc., and achieves the advantages of industrial production, mild reaction conditions, and control The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
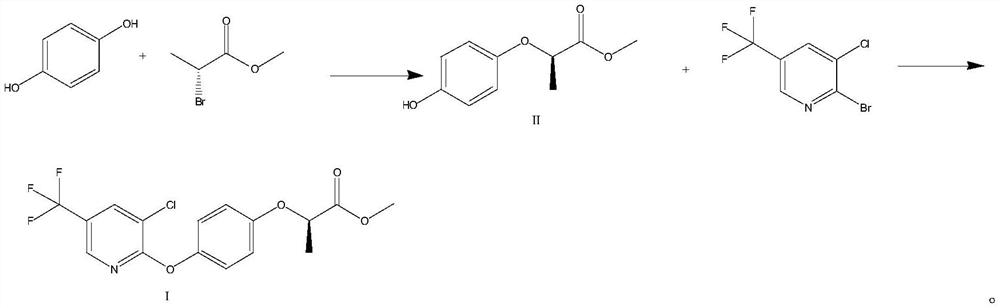
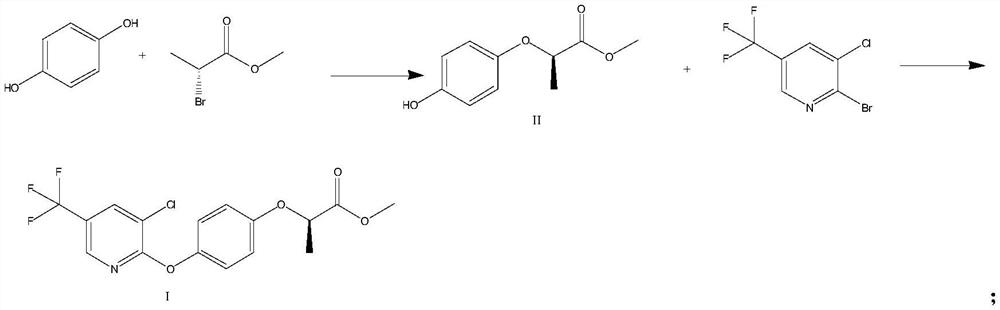
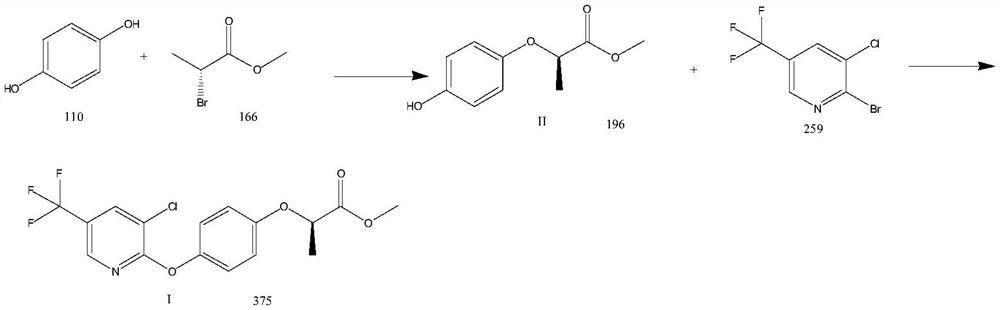
[0029] (1) Methyl (R)-2-(4-hydroxyphenoxy)propionate (Formula II)
[0030] Add hydroquinone (11g, 100mmol), methyl (R)-2-bromopropionate (16.6g, 100mmol), potassium carbonate (20.7g, 150mmol) and DMF (110mL) in the 500mL three-necked flask, heat up Reaction at 70°C for 8h. The reaction solution was cooled to room temperature, and H was added slowly 2 O (200mL), extracted twice with ethyl acetate (200mL*2), washed with 20% NaCl aqueous solution (100mL), concentrated to give 19.3g of (R)-2-(4-hydroxyphenoxy)propionate methyl ester, Yield 98.5%, HPLC purity 99.2%. ESI-MS(m / z):197.08[M+H] + .
[0031] (2) (R)-2-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenoxy)propionic acid methyl ester (Formula I)
[0032] Add (R)-2-(4-hydroxyphenoxy)methyl propionate (9.8g, 50mmol), 3-chloro-2-bromo-5-trifluoromethylpyridine (15.5g , 60mmol), copper powder (160mg, 2.5mmol) and DMF (100mL), heated to 80°C for 12h. After the reaction was completed, the reaction solution...
Embodiment 2
[0034] (1) Methyl (R)-2-(4-hydroxyphenoxy)propionate (Formula II)
[0035] Add hydroquinone (11g, 100mmol), methyl (R)-2-bromopropionate (18.2g, 110mmol), potassium carbonate (20.7g, 150mmol) and DMF (110mL) in the 500mL three-necked flask, heat up Reaction at 70°C for 8h. The reaction solution was cooled to room temperature, and H was added slowly 2 O (200mL), extracted twice with ethyl acetate (200mL*2), washed with 20% NaCl aqueous solution (100mL), concentrated to give 19g of (R)-2-(4-hydroxyphenoxy)methyl propionate, and Yield 96.9%, HPLC purity 99.0%. ESI-MS(m / z):197.08[M+H] + .
[0036] (2) (R)-2-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenoxy)propionic acid methyl ester (Formula I)
[0037] Add (R)-2-(4-hydroxyphenoxy)methyl propionate (9.8g, 50mmol), 3-chloro-2-bromo-5-trifluoromethylpyridine (14.2g , 55mmol), copper powder (160mg, 2.5mmol) and DMF (100mL), heated to 80°C for 12h. After the reaction was completed, the reaction solution was cooled to r...
Embodiment 3
[0039] (1) Methyl (R)-2-(4-hydroxyphenoxy)propionate (Formula II)
[0040] Add hydroquinone (11g, 100mmol), methyl (R)-2-bromopropionate (16.6g, 100mmol), potassium carbonate (20.7g, 150mmol) and DMF (110mL) in the 500mL three-necked flask, heat up Reaction at 60°C for 11h. The reaction solution was cooled to room temperature, and H was added slowly 2 O (200mL), extracted twice with ethyl acetate (200mL*2), washed with 20% NaCl aqueous solution (100mL), concentrated to give 18.8g of (R)-2-(4-hydroxyphenoxy)propionate methyl ester, Yield 95.9%, HPLC purity 99.0%. ESI-MS(m / z):197.08[M+H] + .
[0041] (2) (R)-2-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenoxy)propionic acid methyl ester (Formula I)
[0042] Add (R)-2-(4-hydroxyphenoxy)methyl propionate (9.8g, 50mmol), 3-chloro-2-bromo-5-trifluoromethylpyridine (15.5g , 60mmol), copper oxide (198mg, 2.5mmol) and DMF (100mL), heated to 80°C for 12h. After the reaction was completed, the reaction solution was cooled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com