Patient selection to enhance anti-tumor immunity in cancer patients
A technology for cancer and patients, applied in anti-tumor drugs, immunoglobulins, antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0233] This article provides the following implementations:
[0234] 1. A method of selecting a patient or patient population for cancer therapy comprising administering a CDK4 / 6 inhibitor with chemotherapy in a manner that increases the patient's progression-free survival or overall survival, the method comprising:
[0235] (i) determine whether the patient's cancer has a surrounding microenvironment conducive to immune regulation;
[0236] (ii) determine whether the chemotherapy regimen induces an immune-mediated response such as immunogenic cell death, and if both (i) and (ii) are yes, then
[0237] (iii) administering an effective amount of a CDK4 / 6 inhibitor selected from compound I, II, III, IV or V or a pharmaceutically acceptable salt thereof,
[0238]
[0239]
[0240] where R is C(H)X, NX, C(H)Y or C(X) 2 ,
[0241] Wherein X is linear, branched or cyclic C 1 to C 5 Alkyl groups, including methyl, ethyl, propyl, cyclopropyl, isopropyl, butyl, sec-butyl, te...
Embodiment 1
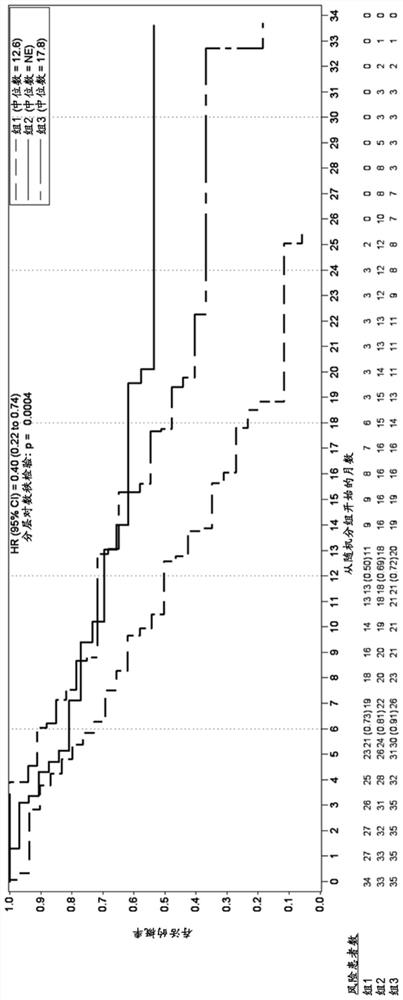
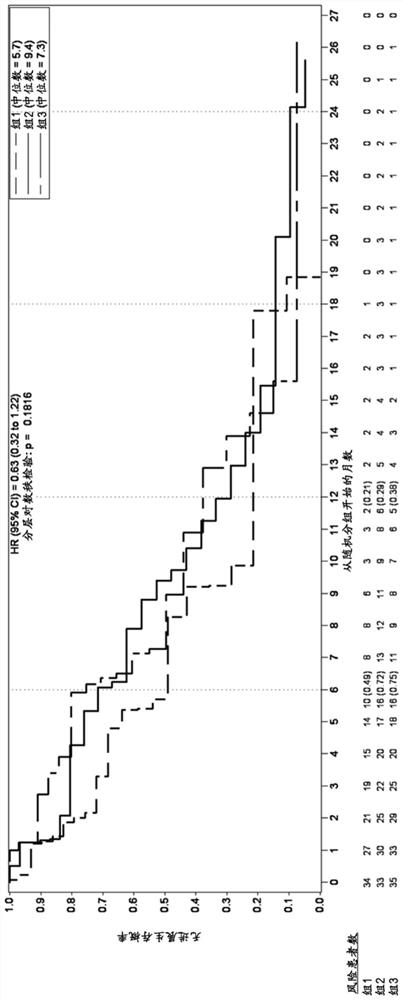
[0459] Example 1. Trelacitinib improves overall survival and progression-free survival in human patients with metastatic triple-negative breast cancer receiving gemcitabine and carboplatin.
[0460] Research design
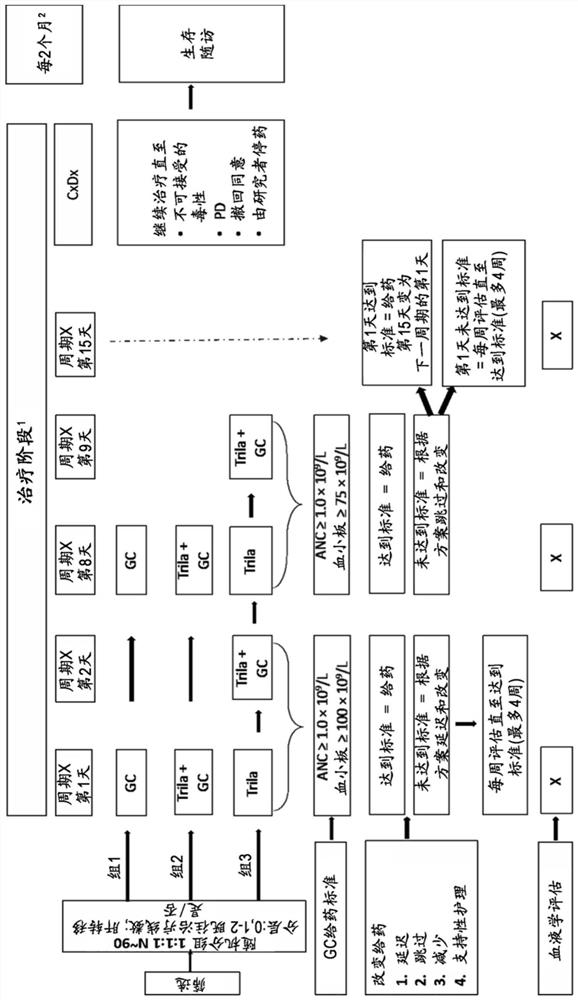
[0461] A multicenter, randomized, open-label, phase 2 study was developed to investigate once-daily administration of triraxime in combination with gemcitabine (IV, 1000 mg / m2) plus carboplatin (IV, AUC-2) (G / C) therapy Safety, Tolerability, Efficacy and PK (G1T28-04) of Nicilin (IV, 240mg / m2) in patients with metastatic TNBC. Patients were randomly assigned (1:1:1 fashion) to 1 of 3 groups:
[0462] Group 1: G / C therapy (days 1 and 8 of a 21-day cycle);
[0463] Group 2: G / C therapy (Days 1 and 8) plus IV administration of Trecitinib on Days 1 and 8 of a 21-day cycle;
[0464] Group 3: G / C therapy (days 2 and 9) plus IV administration of trecicillib on days 1, 2, 8 and 9 of a 21-day cycle;
[0465] Trelacitinib was administered intravenously prior to GC inf...
Embodiment 2
[0524] Example 2: Ayers immune score analysis
[0525] According to Ayers et al., IFN-γ-related mRNA Profile Predicts Clinical Responseto PD-1 Blockade, J Clin Invest. 2017127 (8) 2930-2940 analysis from participating in the clinical trial described in Example 1 (by Q 2 Solutions (Morrisville, NC) assayed tumor samples from patients to determine their Ayers immune score. Data were processed using RNA Access and subjected to FPKM normalization before log10 transformation and averaging.
[0526] 89 samples were analyzed. Computed signature scores for the IFN-γ signature and the extended immune signature were unimodal in distribution and used median scores to define 'high' and 'low' categories. Figure 8A The distribution of the Ayers IFN-γ signature among the 89 samples tested is shown. Figure 8B The distribution of the Ayers expanded immune signature among the 89 samples tested is shown.
[0527] Based on data obtained in the G1T28-04 clinical trial described in Example 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com