Donor pig for xenotransplantation
A technique of xenotransplantation, donor pig, application of tissue and/or cells to avoid xenograft rejection, selection of pigs lacking infectious porcine endogenous retroviruses, enhancement of donor organs, capable of addressing genetic damage to animals Consequences and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0169] This example describes a method for the analysis of PERV content in wild boar by whole genome sequencing.
[0170] 1.1 Method
[0171] Wild boar male 1 is a male AI pig known as pathogen free (DPF) and was identified as PERV-C negative by PCR-based testing.
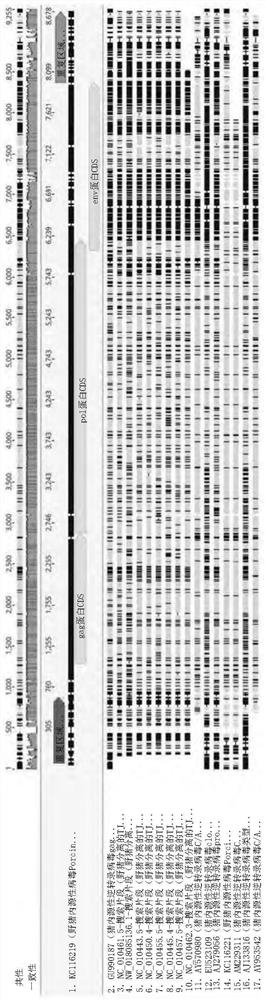
[0172] Complete genome sequencing of wild boar male 1 was performed by Macrogen Inc. under contract. Binary Alignment Map (BAM) files (showing filtered DNA sequence reads mapped to a reference genome with duplicate reads removed) and variant calling format (VCF) files (shown by analysis) were obtained from Macrogen Inc. DNA sequence variants identified from information obtained from aligned reads) and used for further analysis. Regions without coverage from the consensus sequence were replaced with the letter "N" using BCF tools (BCFtools). Geneious was used to BLAST the wild boar male 1 genome to the entire PERV-C sequence of KC116219 (8678bp; NCBI reference sequence).
[0173] 1.2 Results
[0174] 1.2.1 Geno...
Embodiment 2
[0197] This example describes another example of analysis of PERV content in wild boar by whole genome sequencing.
[0198] 2.1 Method
[0199] Wild boar male 2 is an AI male pig known as pathogen free (DPF) and was identified as PERV-C positive by PCR-based testing. Whole-genome sequencing of wild boar male 2 was performed as described in Example 1, Section 1.2.
[0200] 2.2 Results
[0201] 2.2.1 Genome sequencing and identification of PERV sequences
[0202] A total of 25 queries covering more than 50% of the genomic region were detected (KC116219)( Figure 8 ), only 8 covered more than 90%. These were selected as candidates for potential functional PERVs for further analysis (Table 3).
[0203] Table 3. Genomic regions of wild boar male 2 covering more than 90% of the PERV-C sequence (KC116219).
[0204]
[0205] The 8 fragments were combined with variants A, B and C (variant C: KC116219, KC116221, AM229311; recombinant variant A / C: AY570980, AY953542; variant A: AJ...
Embodiment 3
[0221] This example describes the generation and characterization of the NZK1 cell line derived from Auckland Island (AI) porcine kidney.
[0222] 3.1 Method
[0223] 3.1.1 Isolation of kidney-derived AI porcine cells
[0224] Kidney-derived primary AI porcine cells were isolated from tissue samples from two individual animals. One was a male neonatal piglet (#18 / PA007) that died unexpectedly shortly after birth on February 6, 2018, and the resulting cell line was denoted NZK1.
[0225] Kidney cells were isolated based on the method from Richter et al. (2012). Briefly, 1 cm from the renal cortex and medulla were first washed 3 Cube the tissue, then centrifuge after mincing. Afterwards, the tissue was resuspended in 15 ml of Hank's buffered saline solution containing 0.1% (w / v) collagenase (type I or II; Invitrogen) and incubated at 37°C with stirring for 1 to 1.5 h. The supernatant containing kidney cells was filtered through a 100 μm mesh, washed with Dulbecco's modified...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com