Compositions and methods for treating lupus nephritis
A technology for lupus nephritis and lupus, applied in the field of compositions and methods for treating lupus nephritis, capable of solving problems such as unimproved clinical results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0353] The present invention will be more fully understood by reference to the following examples. However, they should not be construed as limiting the scope of the invention. It should be understood that the examples and embodiments described herein are for illustrative purposes only, and that various modifications or changes thereof will be suggested to those skilled in the art, and will be included within the spirit and scope of this application and the scope of the appended claims. within range.
example 1
[0354] Example 1: Obinutuzumab plus mycophenolate mofetil and corticosteroids in the treatment of proliferative lupus nephritis
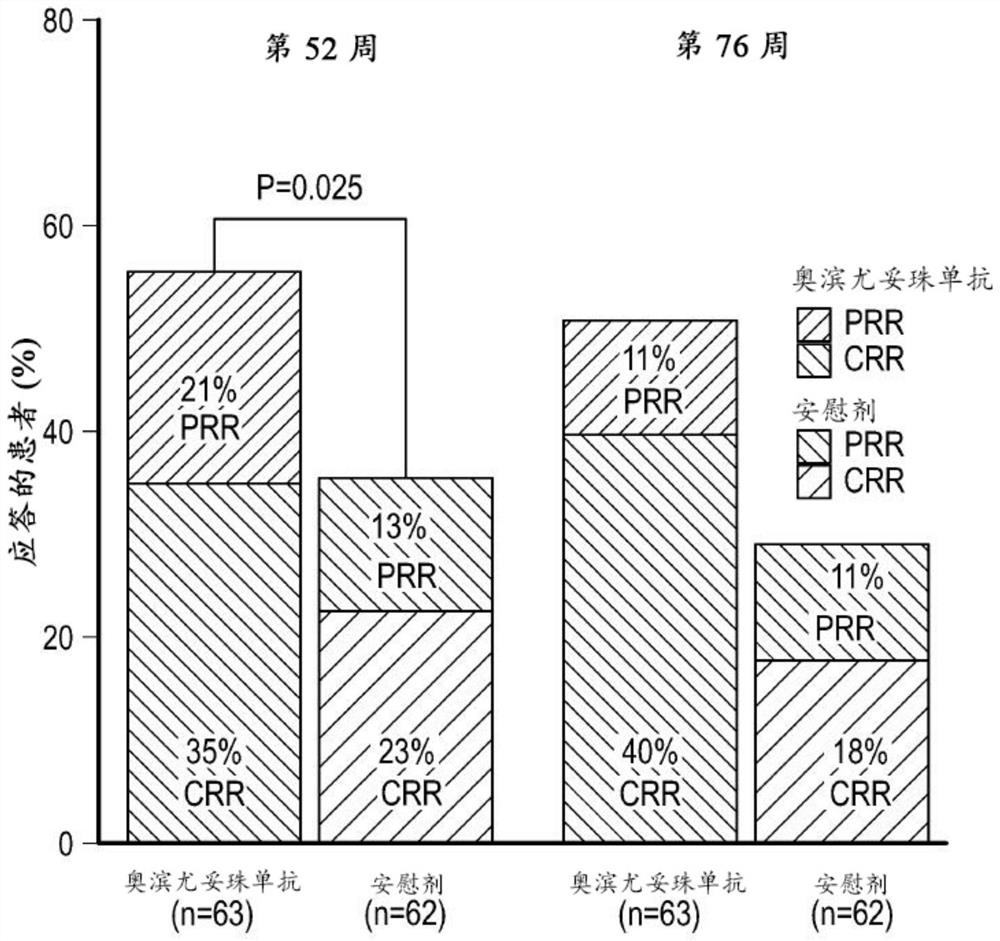
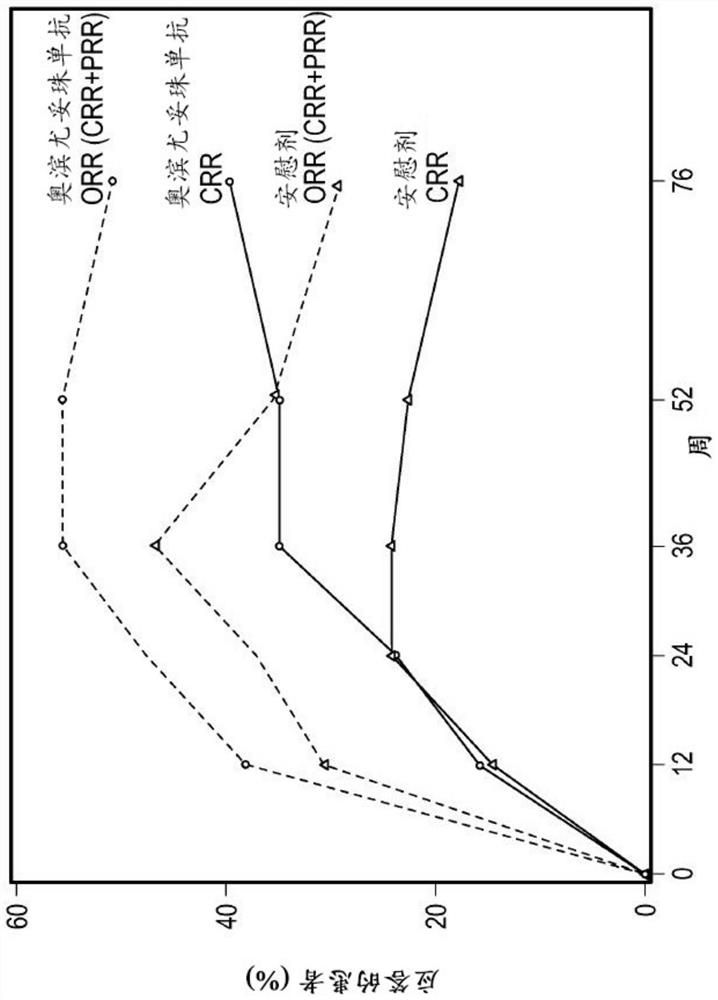
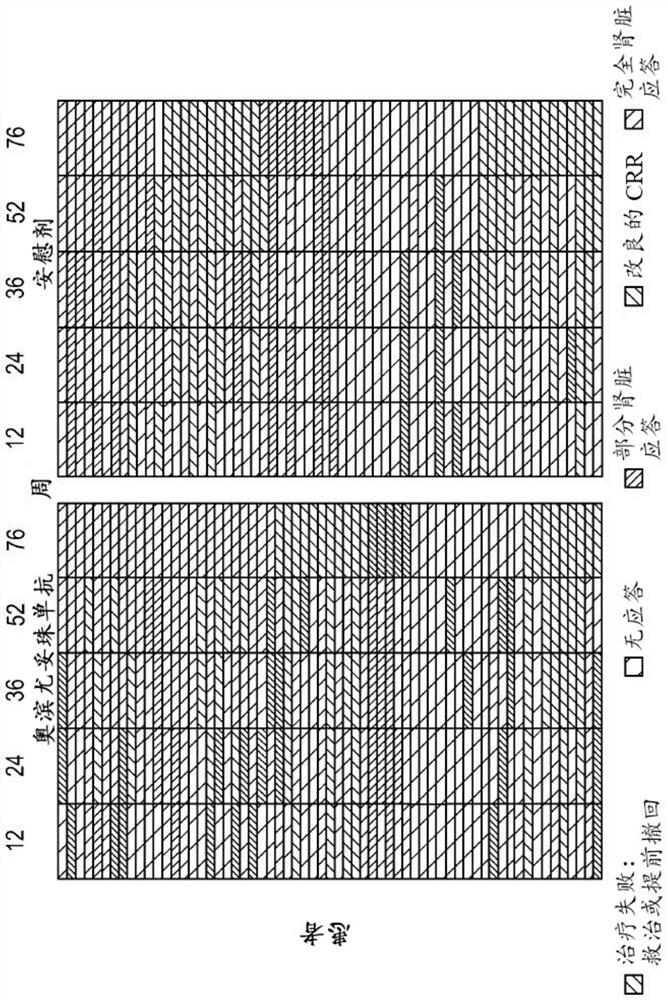
[0355] B cells are central to the pathogenesis of lupus nephritis, but randomized controlled trials of type I anti-CD20 antibodies have failed to demonstrate superiority to standard of care alone. Obinutuzumab, a glycoengineered type II anti-CD20 monoclonal antibody, induces greater B cell depletion compared with type I anti-CD20 antibodies. Obinutuzumab was compared with placebo treatment in patients with proliferative lupus nephritis treated with mycophenolate mofetil and corticosteroids.
[0356] Obinutuzumab was compared with placebo in a phase 2, multicenter, randomized, double-blind trial (NOBILITY) in patients with proliferative lupus nephritis treated with mycophenolate mofetil and corticosteroids. The efficacy of the drug, the results are as follows.
[0357] Materials and Methods
[0358] 126 patients were enrolled at 43 sites in Nort...
example 2
[0436] Example 2: A Modified Obinutuzumab Dosing Regimen for Combining Mycophenolate Mofetil and Corticosteroids in the Treatment of Proliferative Lupus Nephritis.
[0437] The study described in Example 1 showed that a dosing regimen of obinutuzumab infused at 1000 mg at weeks 0, 2, 24, and 26 in combination with standard-of-care immunosuppression demonstrated that at weeks 52 and 76 It will have curative effect and acceptable safety in patients with lupus nephritis (LN). This example describes how a modeling approach was used to predict the expected obinutuzumab following a 1000 mg dosing regimen combined with mycophenolate mofetil and a corticosteroid infusion at weeks 0, 2, 24, 26, and 52. Anti-PK.
[0438] Population Pharmacokinetic Model
[0439] Based on the data presented in Example 1 a population pharmacokinetic (PK) model was developed. The analytical data set used to develop the PK model included 658 obinutuzumab post-dose serum concentration values from 63 pat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com