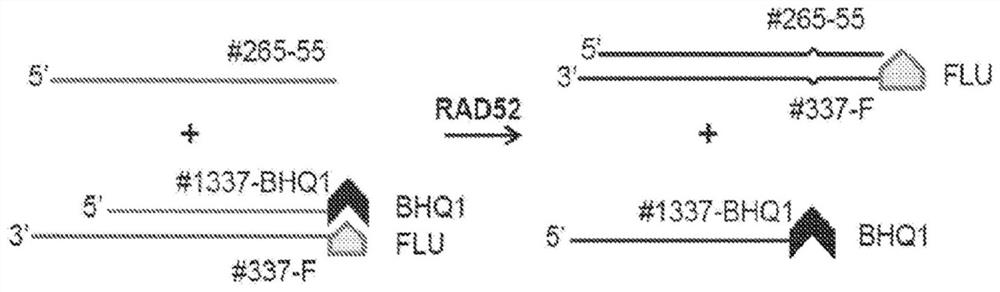
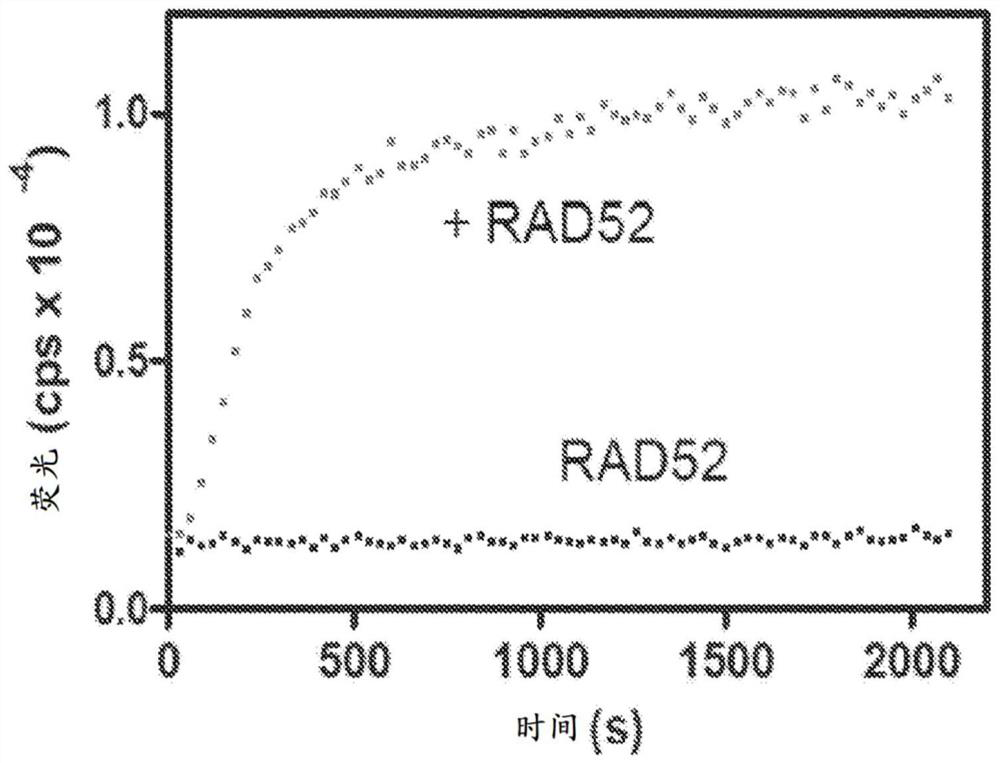
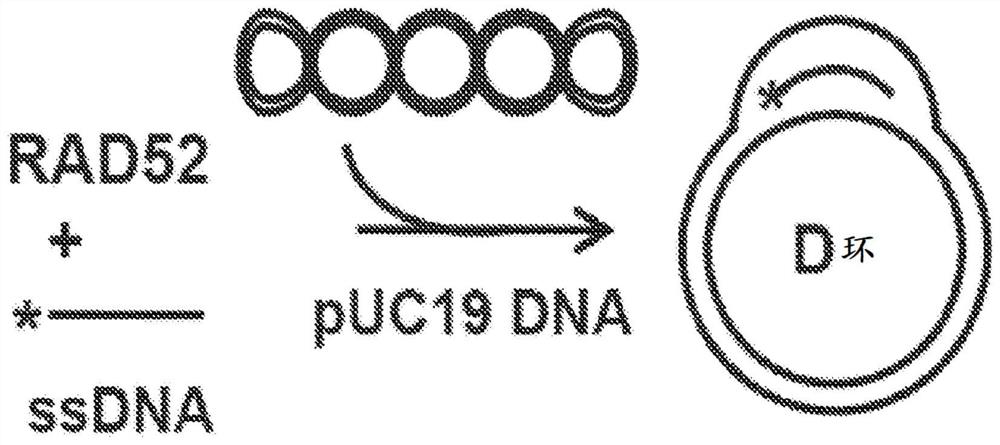
Quinoline inhibitors of RAD52 and methods of use
A technology of N-R2 and solvates, applied in the field of small molecule modulators, can solve problems such as lethality and interruption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0980] Example 1. Synthesis of Compounds of the Disclosure
[0981] General Synthetic Scheme for the Preparation of Compounds of Formula I
[0982] Reagents useful in the preparation of the compounds described herein are either commercially available or prepared by standard procedures described in the literature. Compounds of formula I' or formula I can be produced by one of the following reaction schemes.
[0983] A first aspect of the disclosed method relates to a method of preparing a quinoline having Formula I' or Formula I.
[0984] Compounds of Formula I' or Formula I can be prepared according to the methods outlined in Schemes 1-17.
[0985]
[0986] Scheme 11. Preparation of compounds of Formula I' or Formula I from reactions at the 6-amine group
[0987] Thus, an appropriately substituted compound of formula (A) (a known compound or a compound prepared by a known method) and compound B (a known compound or a compound prepared by a known method), optionally in an o...
Embodiment 2
[1284] Example 2. Cell Assays
[1285] cell line:
[1286] 1. BRCA1-deficient / healthy cell line: UWB1.298 / UWB1.298(BRCA1+)
[1287] 2. BRCA2 deficient / healthy cell line: Capan-1 / Capan-1(BRCA2+)
[1288] Program:
[1289] Capan-1 and Capan-1 (BRCA2+) cells were maintained in IMDM (ATCC) medium containing 20% FBS (GIBCO). UWB1.298 and UWB1.298(BRCA1+) cells were maintained in 48.5% RPMI1640 (ATCC), 48.5% MEGM (Clonetics / Lonza, MEGM kit, CC-3150) and 3% FBS (GIBCO), respectively. The log phase cells were harvested, and 100 μl of the cell suspension was plated in a 96-well plate at a final density of 4000 cells / well. After overnight growth, cells were treated with the indicated concentrations of compounds. Medium containing constant concentrations of compound was refreshed every 3 days until cells were finally lysed with 30 μl / well of Promega CellTiter-Glo reagent and read on a Promega GloMax 96 reader at day 10 (9 days of exposure).
Embodiment 3
[1290] Example 3. Clonogenic Survival Assay
[1291] MDA-MB-436 cells were cultured in RPMI+10% FBS. BRCA-proficient and BRCA-deficient cells were plated at 5,000 cells / well on day 0 in triplicate. Cells were counted on day 4 using trypan blue exclusion on a hemocytometer and immediately plated at a density of 500 cells / well in 6-well plates in RPMI+10% FBS in the clonogenicity assay. After two weeks, colonies were fixed / stained with 0.05% of 10 mg / ml ethidium bromide in 50% ethanol and visualized with an Alphaimager gel imager (Alpha Innotech).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com