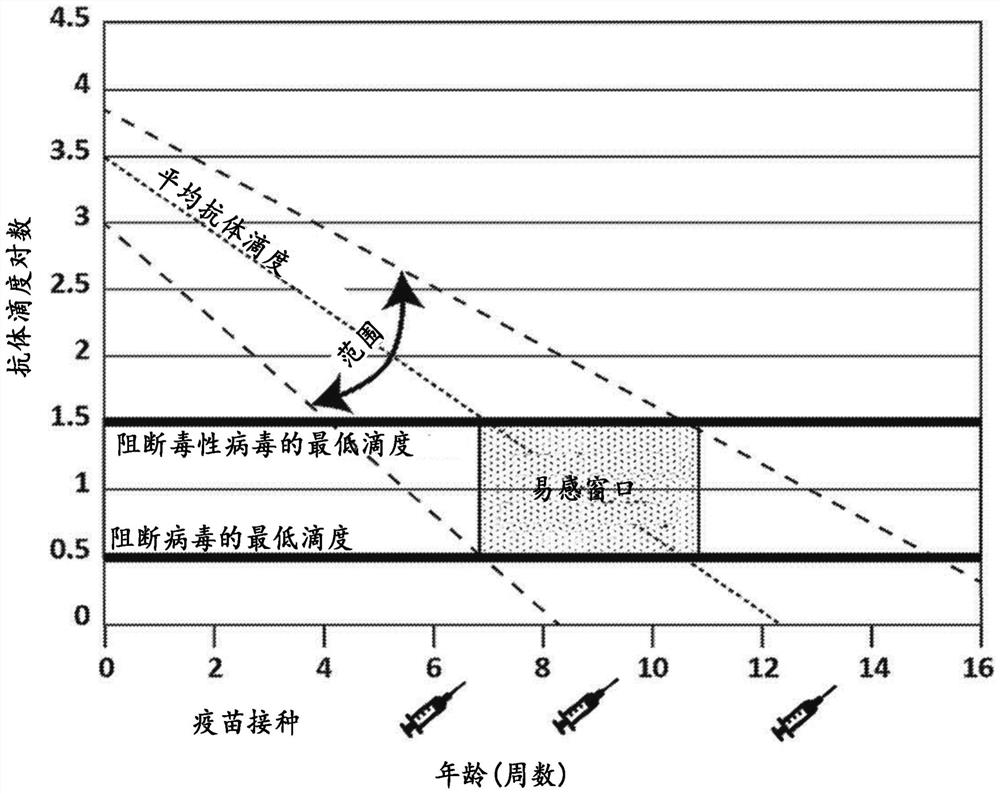
Veterinary parvovirus antibody
A canine parvovirus, feline parvovirus technology, applied in the direction of antibodies, antiviral agents, antibody medical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0199] Pharmaceutical compositions can be stored in lyophilized form. Thus, in some embodiments, the method of preparation includes a lyophilization step. The lyophilized composition can then be reconstituted, usually as an aqueous composition suitable for parenteral administration, prior to administration to dogs, cats or horses. In other embodiments, especially where the antibody is highly stable to thermal and oxidative denaturation, the pharmaceutical composition may be stored as a liquid, i.e. as an aqueous composition, which may be administered directly or with appropriate dilution for dogs, cats or horses. Lyophilized compositions can be reconstituted with sterile water for injection (WFI). Antimicrobial agents (eg, bacteriostats such as benzyl alcohol) may be included. Accordingly, the present invention provides pharmaceutical compositions in solid or liquid form.
[0200] When administered, the pH of the pharmaceutical composition may be in the range of about pH 5...
Embodiment 1
[0224] Preparation of chimeric canine antibodies Mab A and Mab B
[0225] Structures of canine and feline parvovirus complexed with antibody fragments from eight different neutralizing monoclonal antibodies are reported in Hafenstein S. et al., J Virol. 2009 Jun, 83(11):5556-66 analyze. Incomplete amino acid sequence information is provided for rat Mab E and Mab F Fab fragments that exhibit neutralization of canine parvovirus in in vitro assays. The partial amino acid sequences of the variable heavy chain and variable light chain of Mab E are SEQ ID NO. 81 and 82, respectively. The partial amino acid sequences of the variable heavy chain and variable light chain of Mab F are SEQ ID NO. 83 and 84, respectively.
[0226] Three-dimensional protein structure analysis was performed to construct the redesigned first framework regions of the heavy and light chains of Mab E and Mab F. The amino acid sequences of the heavy and light chains of the redesigned monoclonal antibodies des...
Embodiment 2
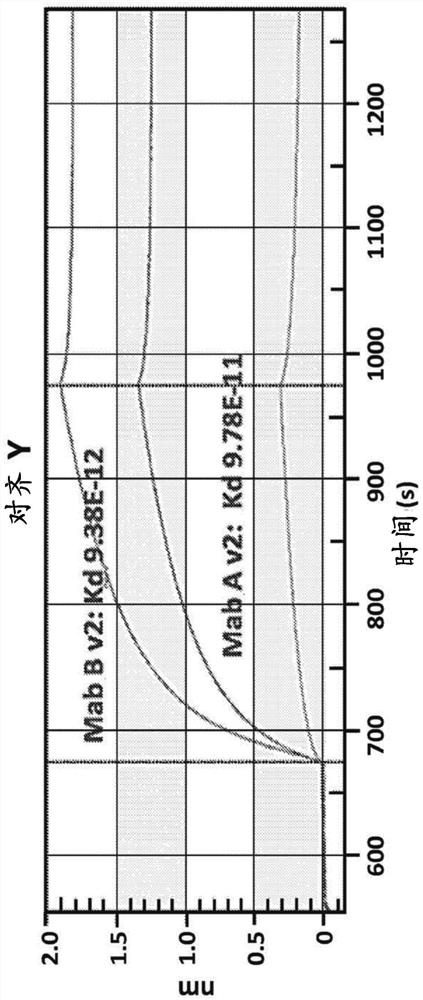
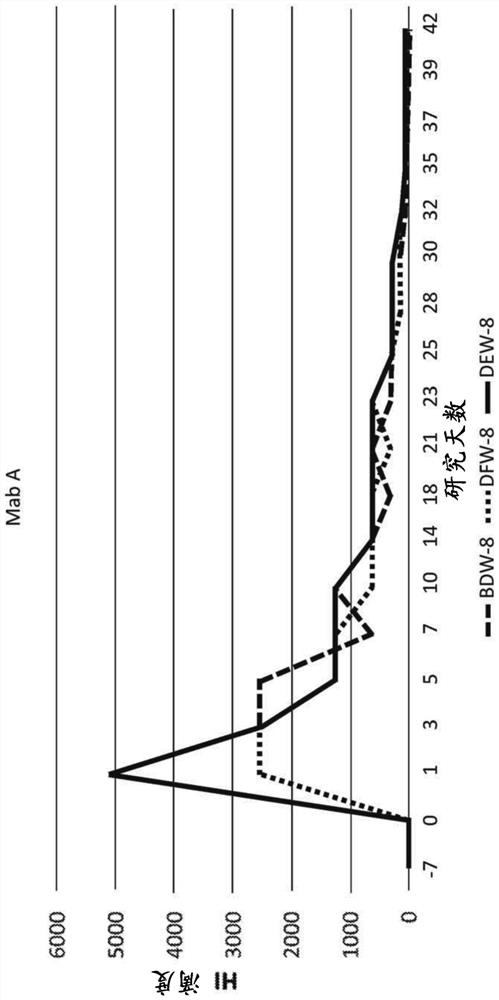
[0230] Chimeric canine antibody Mab A and Mab B activities
[0231] Both purified chimeric canine Mab A and Mab B (formulated at 200 μg / mL in PBS (pH 7.2)) were used to assess anti-canine parvovirus activity using a hemagglutination inhibition (HI) assay. Assays were performed at the Companion Animal Vaccine and Immunodiagnostic Services Laboratory at the University of Wisconsin substantially as described by Carmichael et al., Am J of VetRes. 1980 May, 41(5):784-91. Serial dilutions of chimeric canine Mab A and chimeric canine Mab B samples incubated with CPV were prepared. Then add porcine red blood cells. Canine parvovirus-induced agglutination of porcine erythrocytes. Both chimeric canine Mab A and chimeric canine Mab B prevented agglutination. Both antibodies yielded a CPV-2b HI of 40960, while an irrelevant canine IgG yielded a HI of <20. Most vaccinated dogs are thought to have an antibody HI of about 1280.
[0232] In addition, the activity of antibodies to prevent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com