Heat-resisting xylanase and gene coding the xylanase
A xylanase, gene technology, applied in the field of xylanase, can solve problems such as industrial application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Extraction of Thermotoga maritima Genomic DNA
[0026] 20 g of fresh wet bacteria of Thermotoga maritima (Thermotoga maritima MSB8, German Culture Collection, DSM3109) were used to suspend in 10 mL of 50 mM Tris buffer (pH 8.0), and a small amount of lysozyme and 8 mL of 0.25 mM EDTA (pH 8. 0), mix well and place at 37°C for 20min, then add 2mL of 10% SDS, place at 55°C for 5min, extract once with equal volumes of phenol and chloroform respectively, take the last supernatant, add 2 times the volume of ethanol, DNA recovery. Then wash with 70% and absolute ethanol respectively, dissolve the precipitate in 0.5mL TE buffer (pH8.0, 10mMTris, 1mM EDTA), add 10mg / mL RNase 3μL, keep it at 37°C for 1h, and extract with equal volumes of phenol and chloroform respectively. Once, 2 times the volume of ethanol was added to the supernatant, and the DNA was recovered, washed with 70% ethanol and absolute ethanol, vacuum freeze-dried, and dissolved in ultrapure water.
Embodiment 2
[0027] Embodiment 2 Amplification of xylanase gene xynB
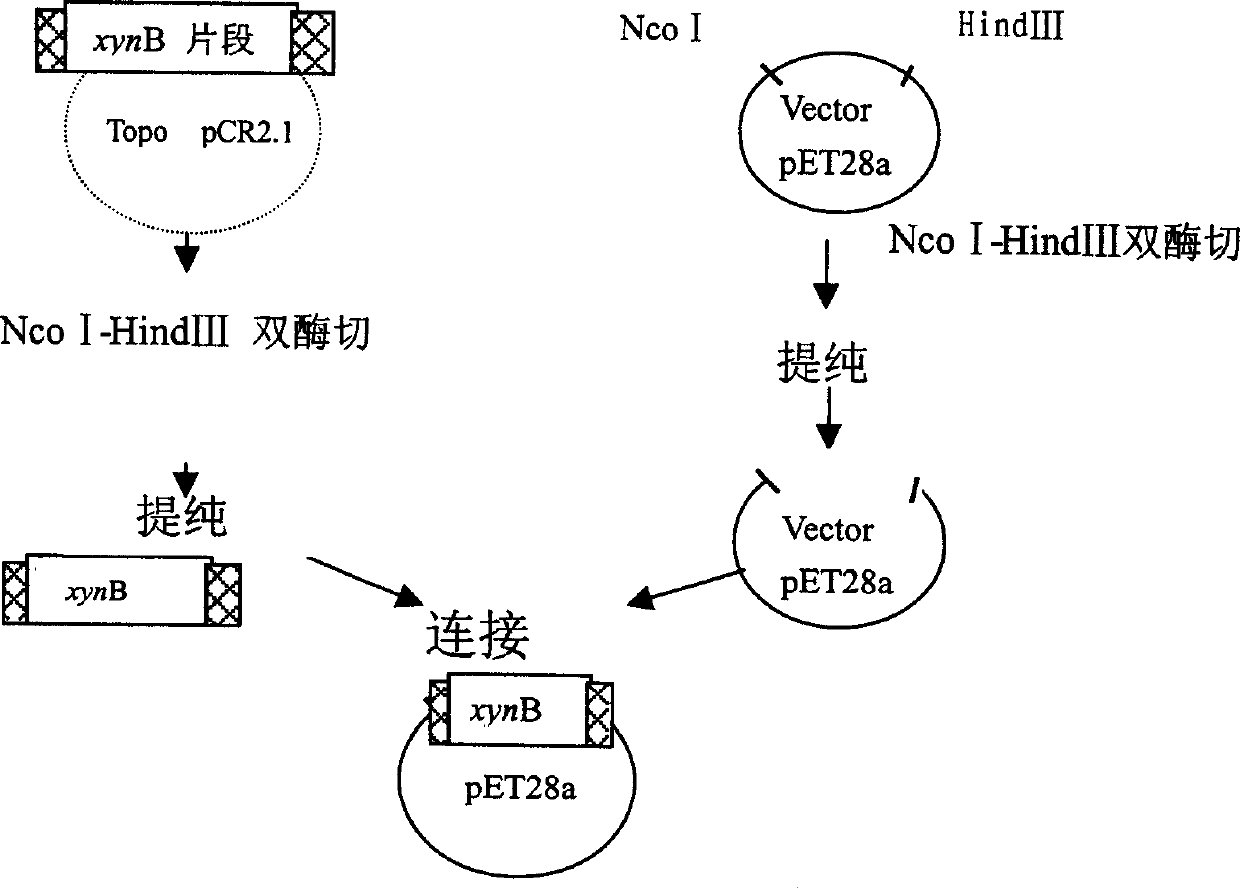
[0028] A pair of primers were designed, and the Nco I-Hind III restriction sites capable of being inserted into the pET28a(+) plasmid (Novagen) were introduced into the primers. The sequences of the primers TM-XynB-Nco I-FWD and TM-XynB-Hind-His-REV used are as follows:
[0029] TM-XynB-Nco I-FWD: 5′-CCATGGAAA TATTACCTTCTGTGTGAT
[0030] TM-XynB-Hind-His-REV: 5′-AAGCTTTCTTTCTTCTATCTTTTTCTCCA
[0031]When designing the TM-XynB-Nco I-FWD forward primer, it is considered that the C-terminus in the expression vector has a 6×His tag, so that the second amino acid from the Nco I restriction site is changed from K to E after translation. This mutation according to the primer design ensures that the target gene is cloned in the open reading frame, with ATG start codon and 6×His tag sequence at the same time, without open reading frame drift.
[0032] Using the genomic DNA of Thermotoga maritima as a template, polymerase was ...
Embodiment 3
[0034] Cloning and DNA sequence determination of embodiment 3 PCR products
[0035] After the PCR amplification reaction, the present invention uses topological TA cloning to clone the xynB gene fragment into the pCR(R)-2.1Topo plasmid vector, and screens colonies with recombinants by colony PCR. Then, use the standard primer sequence on the pCR(R)-2.1Topo plasmid vector as a primer to measure the DNA sequence of the xynB gene, and purify the plasmid containing the xynB gene sequence for further subcloning and expression.
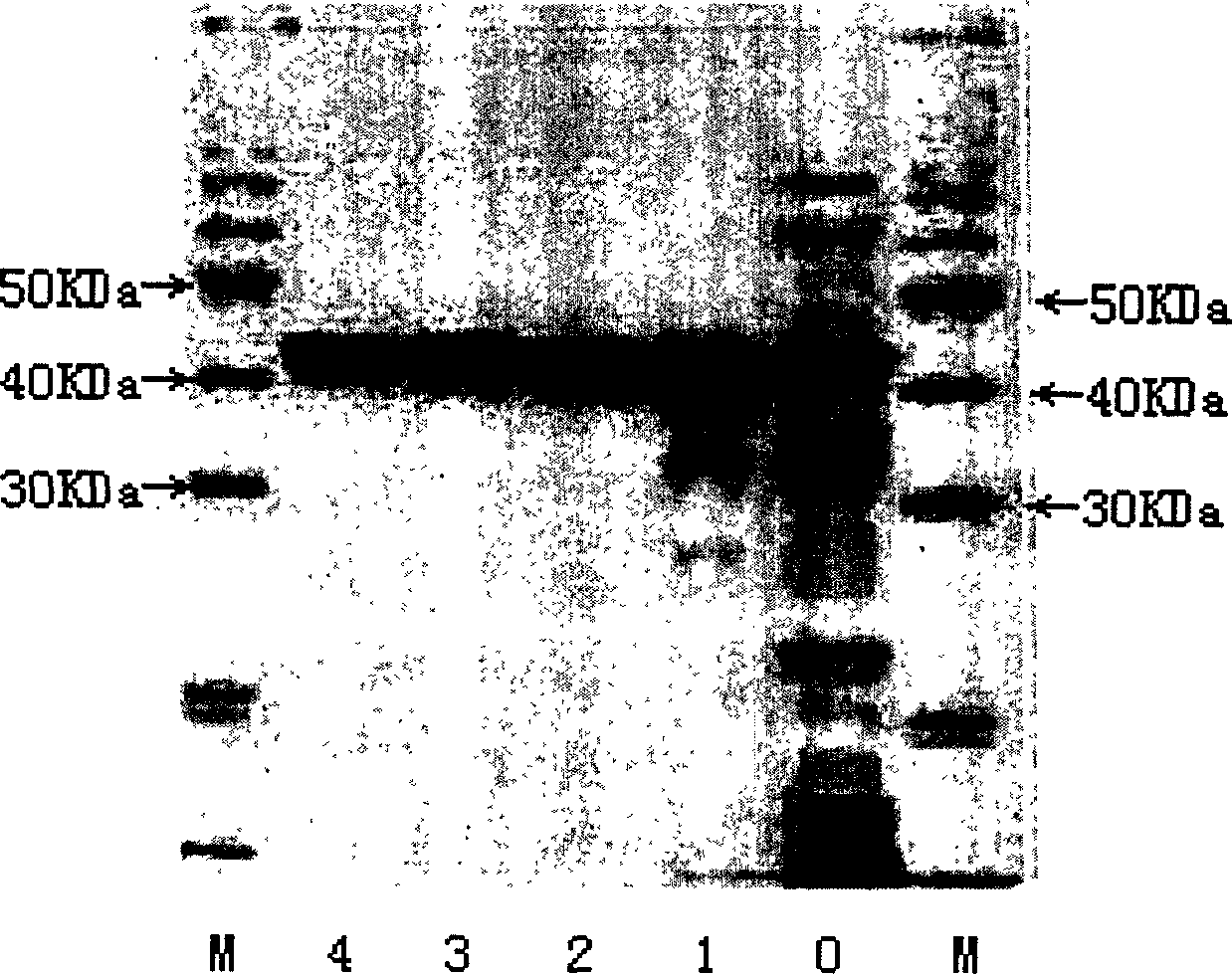
[0036] The present invention adopts terminal termination method (capillary electrophoresis fluorescence method) for sequencing, and the reagent used for sequencing is the Dye Terminor Cycle sequencing kit (Perkin-Elmer, USA). The composition of the sequencing reaction mixture is: Terminator Ready Reaction Mix, 4μl; Plasmid Solution, 3μl; Forward or Reverse primer, 1.5μl; H 2 O, 1.5 μl; the total volume of the reaction mixture was 10 μl. The sequencing cyc...
PUM
| Property | Measurement | Unit |
|---|---|---|
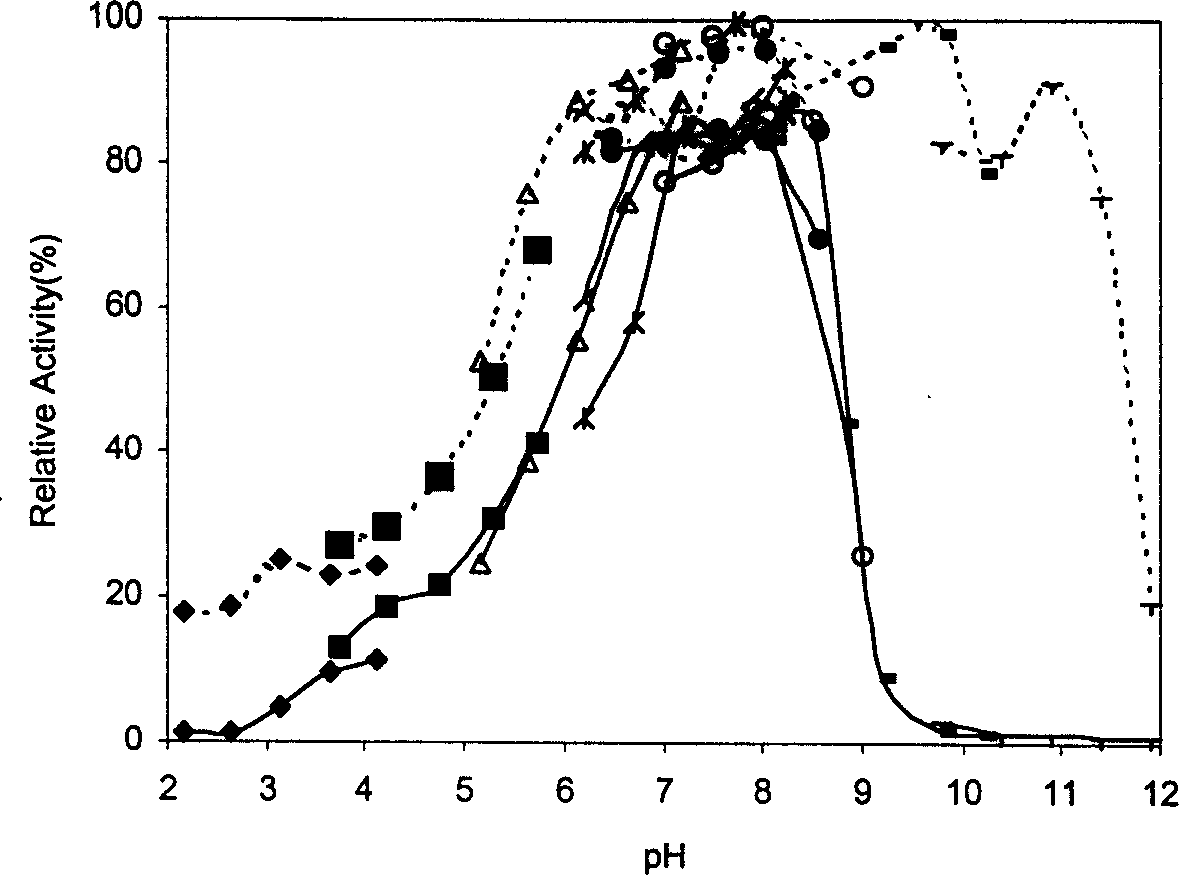
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com