Universal T-cell epitopes for anti-malarial vaccines
An anti-malarial and malaria technology, applied in the field of vaccines with universal T-cell epitopes, can solve the problem of vaccines failing to trigger immune responses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] The following examples are intended to serve as non-limiting illustrations of the invention. Example 1: Anti-malarial vaccines containing MAP
[0092] Studies in mice of different genetic backgrounds have shown that: * Peptide-based vaccines of epitopes (see above) are immunogenic when unadjuvanted (ie when administered alone in phosphate buffered saline).
[0093] Enhanced antibody responses were obtained by adding adjuvants such as alum (Rehydragel, Reheis NJ) or QS21 (CambridgeBiotech, Cambridge MA).
[0094] A typical antimalarial vaccine containing MAP has 1 mg mixed with 100 μg QS21 (T * T1B) 4 map. This vaccine is given by injection under the skin. Example 2: Eliciting CS-specific antibodies in humans
Embodiment 2
[0094] A typical antimalarial vaccine containing MAP has 1 mg mixed with 100 μg QS21 (T * T1B) 4 map. This vaccine is given by injection under the skin. Example 2: Eliciting CS-specific antibodies in humans
[0095] The following studies were performed to examine the immunizing effect of a vaccine containing a universal T-cell epitope on a variety of humans with different genetic backgrounds.
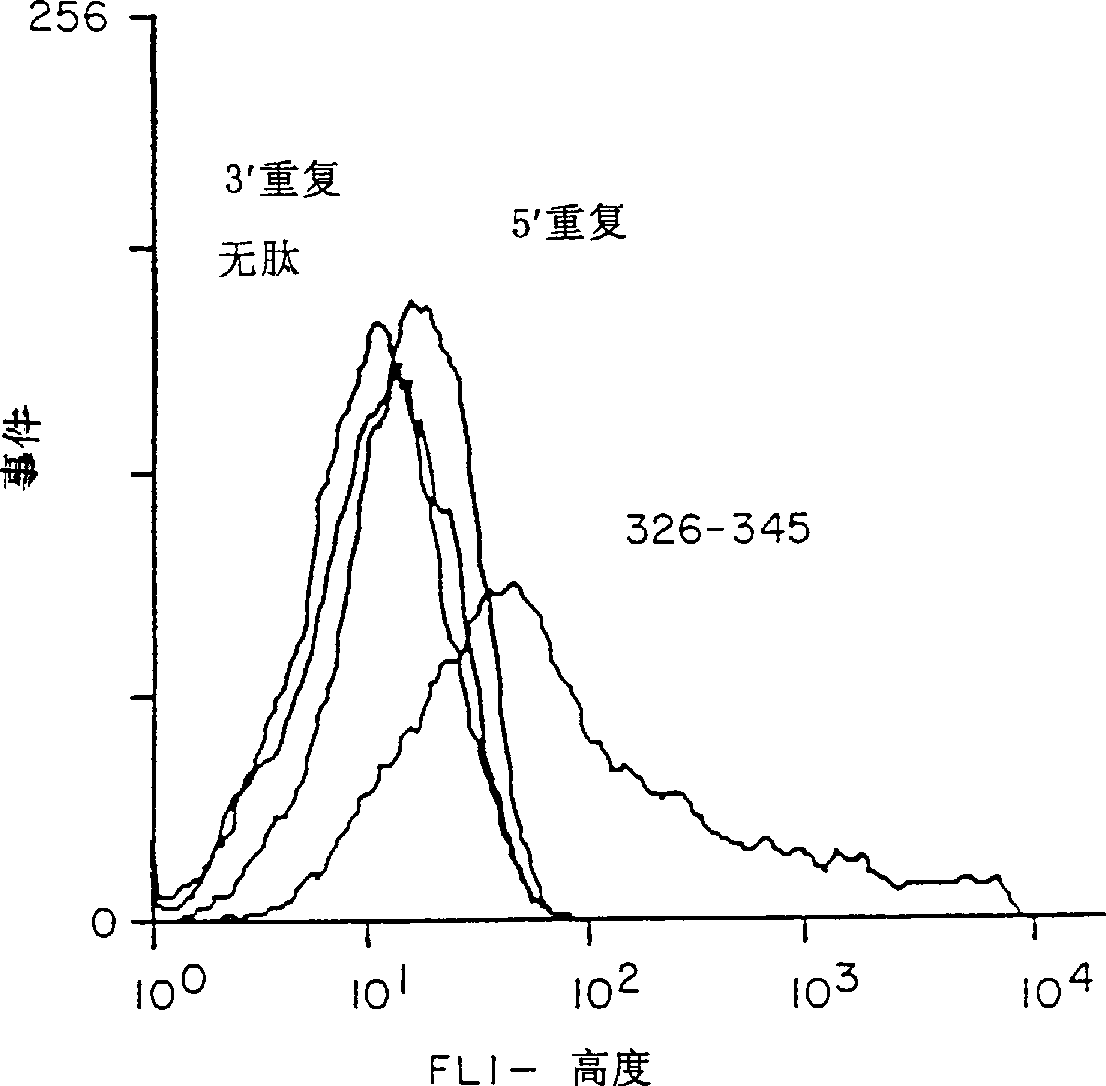
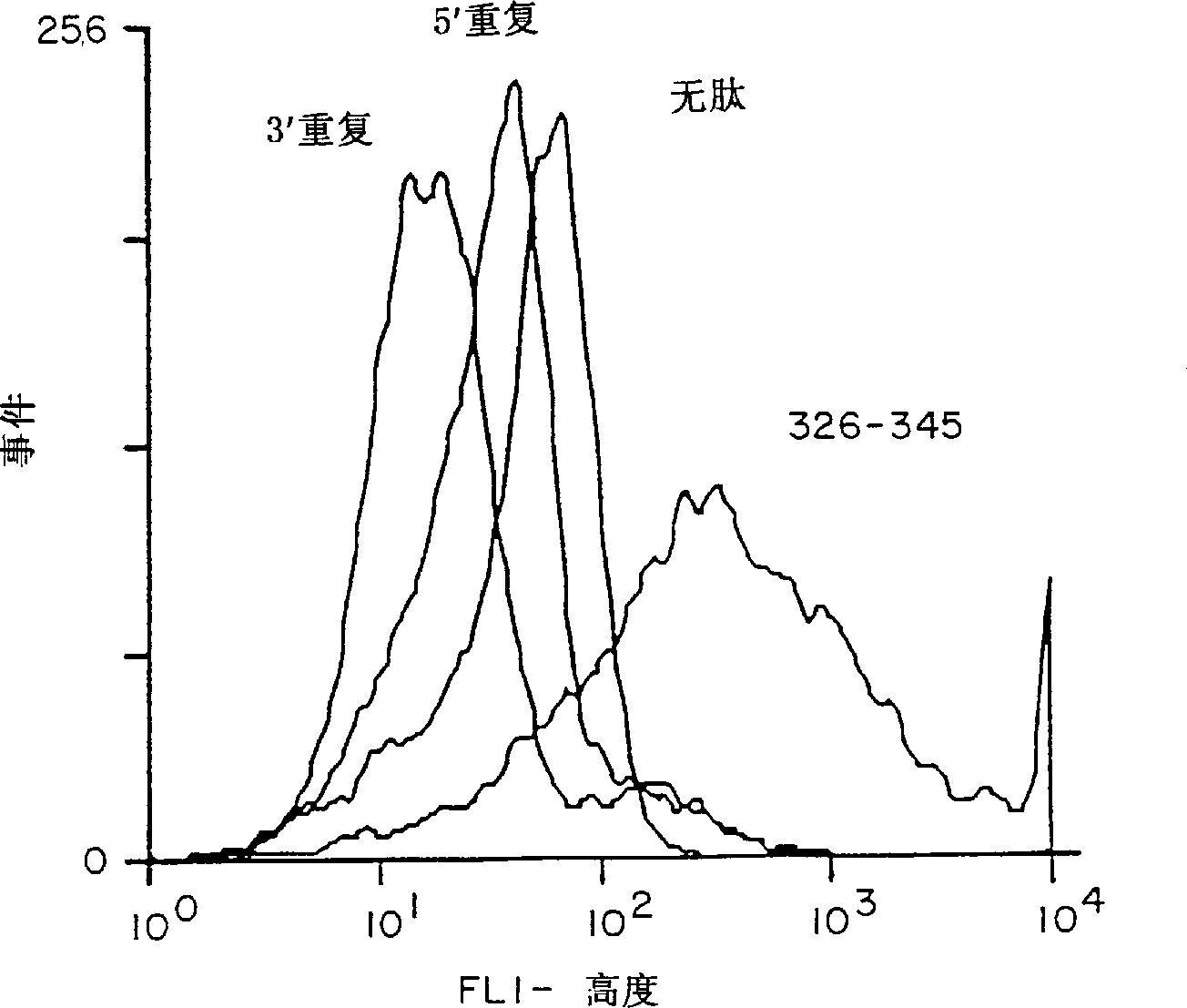
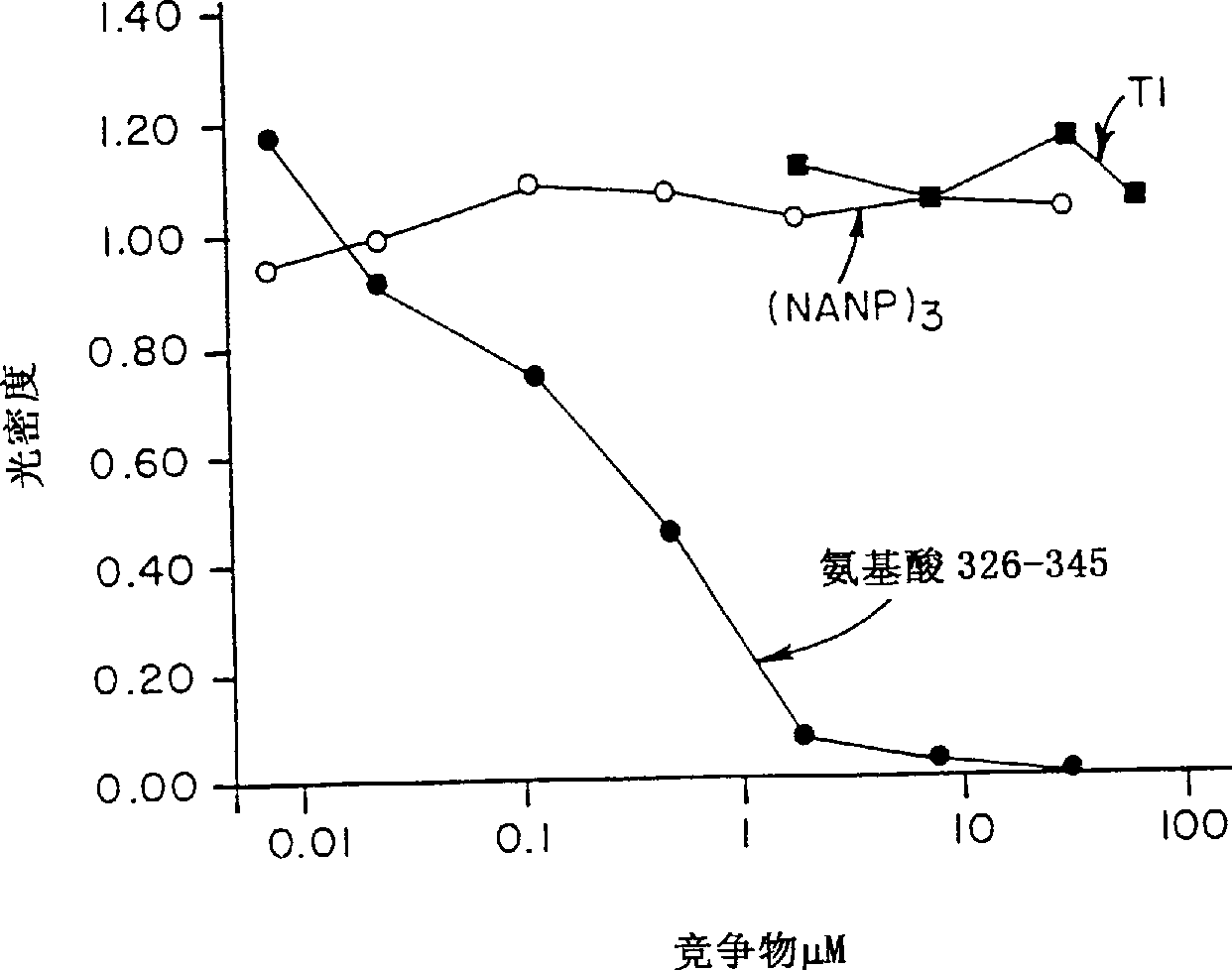
[0096] METHODS: A compound called (T1BT * ) 4 - Polyoxime synthetic malaria vaccine of P3C. The vaccine contains universal T cell epitopes as described above (T * ) and a 28-residue repeat (DPNANPNV) derived from the Plasmodium falciparum CS repeat 3 (NANP) 3 (called T1B) combined. The vaccine also contains a covalently linked synthetic adjuvant tripalmitoylcysteine (Pam 3CyS) attached to the lysine core. Methods for the synthesis of immunogenic polyoxime compositions are disclosed in International Patent Application WO 94 / 25071. for T * Methods for the synthesis of polyox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com