Method and composition for altering a t cell mediated pathology
A composition and cell technology, applied in the field of immunology and immunotherapy, can solve the problems of low production level and difficult production of TCR molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
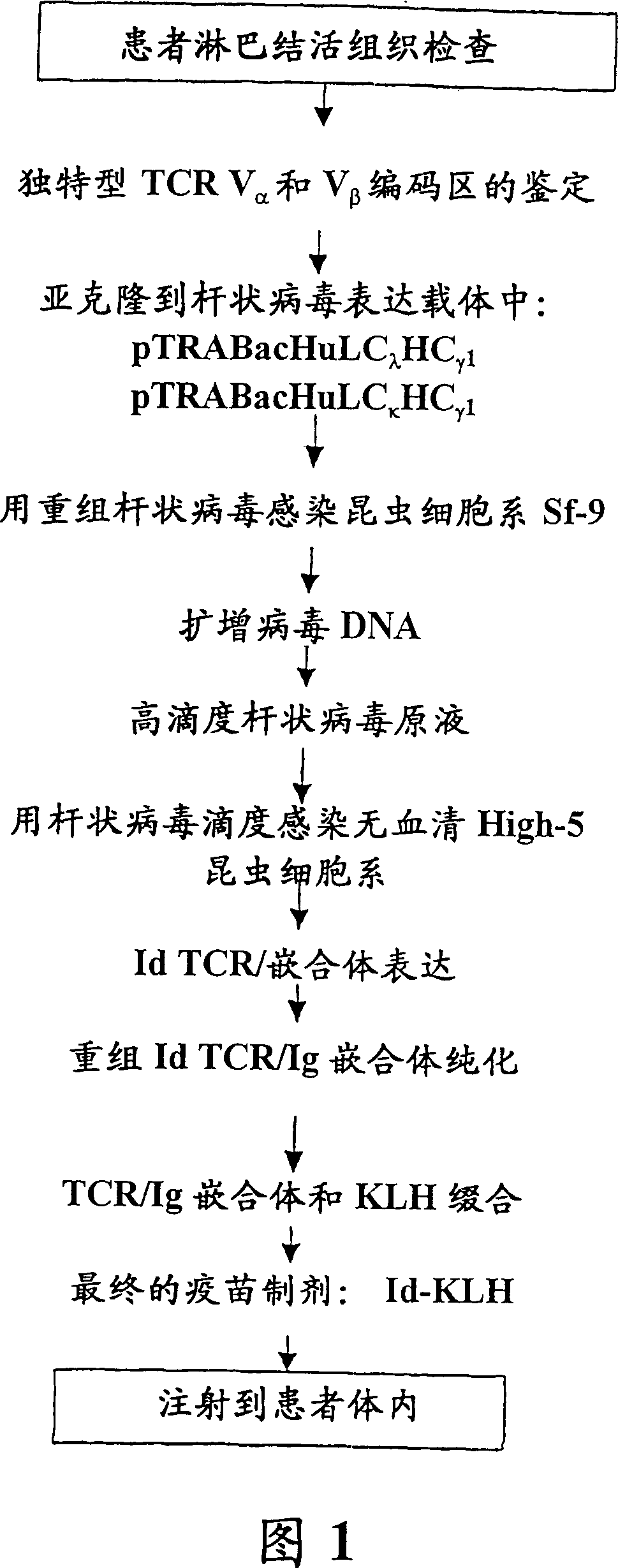
[0159] Example 1. Tissue processing for T-cell lymphoma idiotype (Id) identification and cloning:
[0160] Tumor samples obtained from peripheral lymph nodes were biopsied under sterile conditions according to clinically prescribed methods, and the samples were used to produce patient idiotype-specific recombinant V α and V β - Ig chimeric protein. Store the remaining lymph node biopsy material in a tissue cell bank in liquid nitrogen for future use.
[0161]Cell Isolation: Single cell suspensions of patient lymph node biopsy samples were obtained by forcing the patient biopsy lymphoma tissue through a disposable 0.38 mm steel mesh and simultaneously immersed in sterile PBS. Dispersed cells were washed twice with PBS, then resuspended and counted. A 10% ratio of the cells was processed for total RNA extraction and the remaining cells were stored in liquid nitrogen after resuspension in RPMI 1640 tissue culture medium containing 30% fetal bovine serum and 10% DMSO. All clin...
Embodiment 2
[0169] Example 2. Baculovirus expression vector pTRABacV containing immunoglobulin heavy chain and light chain constant regions α HC γ1 and pTRABacV β HC γ1 build
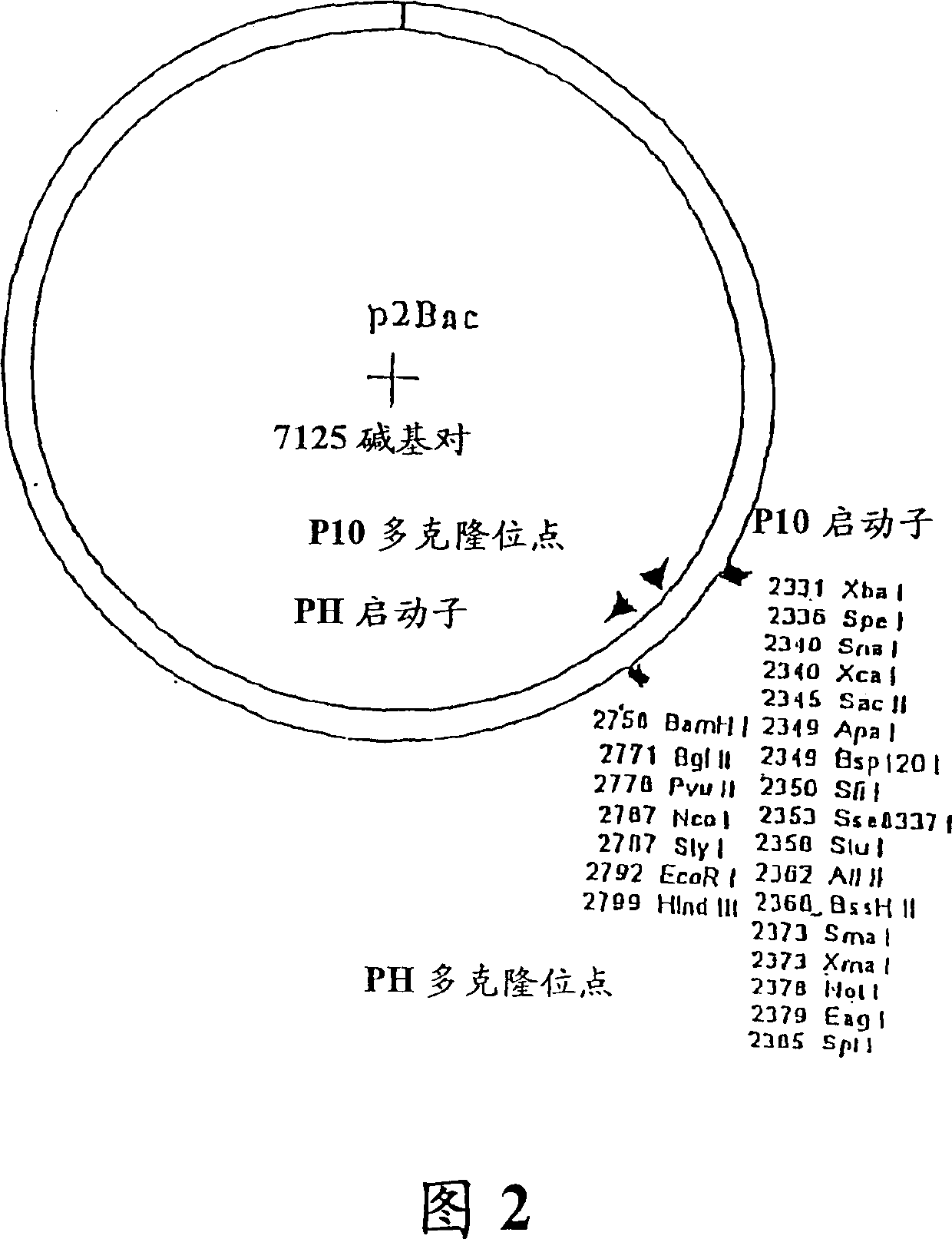
[0170] Cloning of the secretion signal sequence into p2Bac: pTRABacHuLC κ HC γ1 and pTRABacHuLC λ HC γ1 The base vector for the construct was p2Bac (Figure 2, SEQ ID NO: 5, Invitrogen, Carlsbad, CA). The two secretion signal sequences were cloned into the basic vector to construct the first intermediate baculovirus expression vector p2BacM. In summary, vector p2Bac was first modified with complementary oligonucleotides encoding the amino-terminal domain of the honeybee melittin secretion signal sequence positioned to place under the transcriptional control of the baculovirus AcNPV P10 promoter. For cloning of the melittin sequence, 2 μg of p2Bac was digested with Not I and Spe I at 37°C for 4 hours. The linear vector was purified with Qiaex II resin (Qiagen, Chatsworth, CA) after electrophoresis through a ...
Embodiment 3
[0183] Example 3. The patient-derived idiotype V α and / or V β Stranded genes are inserted into expression vectors
[0184] Separate V as described above α and / or V β After the tumor-derived sequence of the chain, an oligonucleotide primer containing the terminal 40 nucleotides of the melittin leader peptide (for V β chain cloning) (SEQ ID NO: 8-ACTAGTTTTT ATGGTCGTGT ACATTTTCTTACATCTATGCG), an oligonucleotide primer containing the terminal 31 nucleotides of the alkaline phosphatase leader peptide (for V α chain clone) (SEQ ID NO: 9-AGGCCTGAGGCTACAGCTCT CCCTGGGC) and containing the corresponding V α or V β Oligonucleotide primers for the first 20 nucleotides of the gene. A reverse oligonucleotide primer complementary to base pairs 4-36 of CA (CA / IgK; SEQ ID NO: 15) and a reverse oligonucleotide primer complementary to base pairs 6-35 of CB from the α or β chain constant region were used. To the oligonucleotide primer (CB / IgG 1 : SEQ ID NO: 14). identified as previously ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com