Antibody affinity purification material and its application
An affinity and antibody technology, applied in the field of biological separation engineering and biomedicine, can solve the problems of intolerance to strong acid and alkali, high price, poor durability and biological safety, etc., and achieve strong alkali resistance, no shedding, biological good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Sepharose 6B is activated with trichlorotriazazine (for operation, refer to "Introduction to Affinity Chromatography" (written by C.R.Lowe; translated by Liu Yuxiu. Science Press, first edition in May 1983), take 5 parts of 200ml each, estimate The number of moles of triazoxide, respectively weighed 5-fold molar excess amino compound (R1) 4-(2-aminoethyl) benzenesulfonamide, N-(3-aminopropyl) imidazole, 4-amino-1-naphthalene Phenol, 2-amino-4,6-dihydroxypyrimidine, dibenzylamine, dissolved in 300ml of DMF, respectively added to the triazoxide activation medium, mixed and stirred at 50 ° C for 24h, saturated NaHCO during the reaction 3 The pH of the solution was maintained at 7-8. After the reaction was completed, the medium was washed with 10 times the volume of DMF (N'N dimethylformamide) and water.
[0019] Then weigh the 5-fold molar excess amino compound (R2) L-serine, N-(3-aminopropyl) imidazole, 3,5-diaminobenzoic acid, γ-aminobutyric acid and dibenzylamine and di...
Embodiment 2
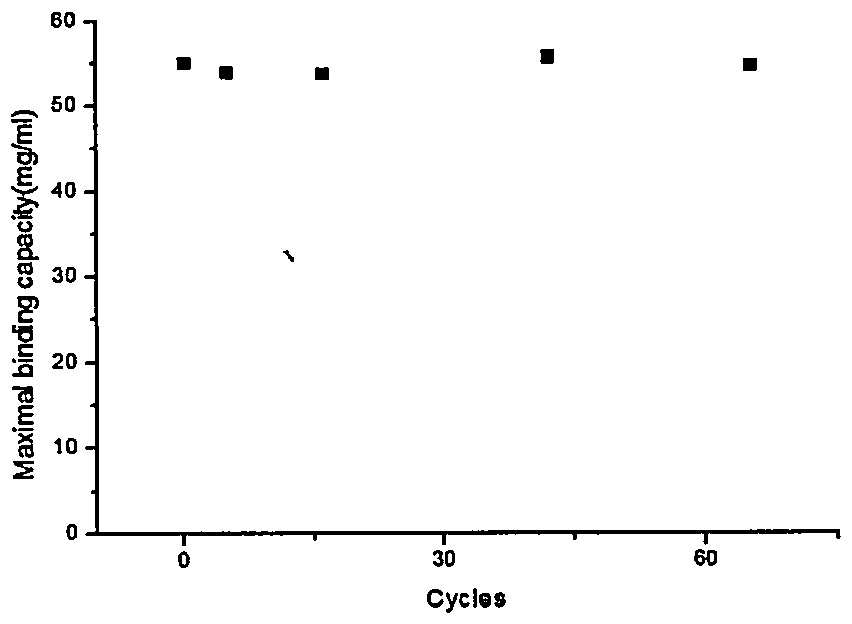
[0021] Physical and chemical property verification of affinity purification materials:
[0022] Affinity purification material C1-2 was packed into a column (160mm×10mm), and the antibody solution was used to continuously inject samples until the UV absorption signal remained unchanged. The original antibody concentration was 1mg / ml, and the sample flow rate was 2ml / min;
[0023] Antibodies were dissolved in 10 mMPBS (pH 7.0, 150 mM NaCl) and equilibrated for 10 column volumes until UV280 stabilized near baseline.
[0024] Record the loading volume of the sample under different flow-through ratios when the flow rate of the sample in the pipeline is 150cm / h, until the protein concentration at the outlet of the column reaches more than 10% of the inlet concentration, stop the injection, and wash the affinity purification material with the loading buffer When the UV absorption value of the column drops to the baseline, the column is washed with Gly-HCl (100mM, pH3), and the total...
Embodiment 3
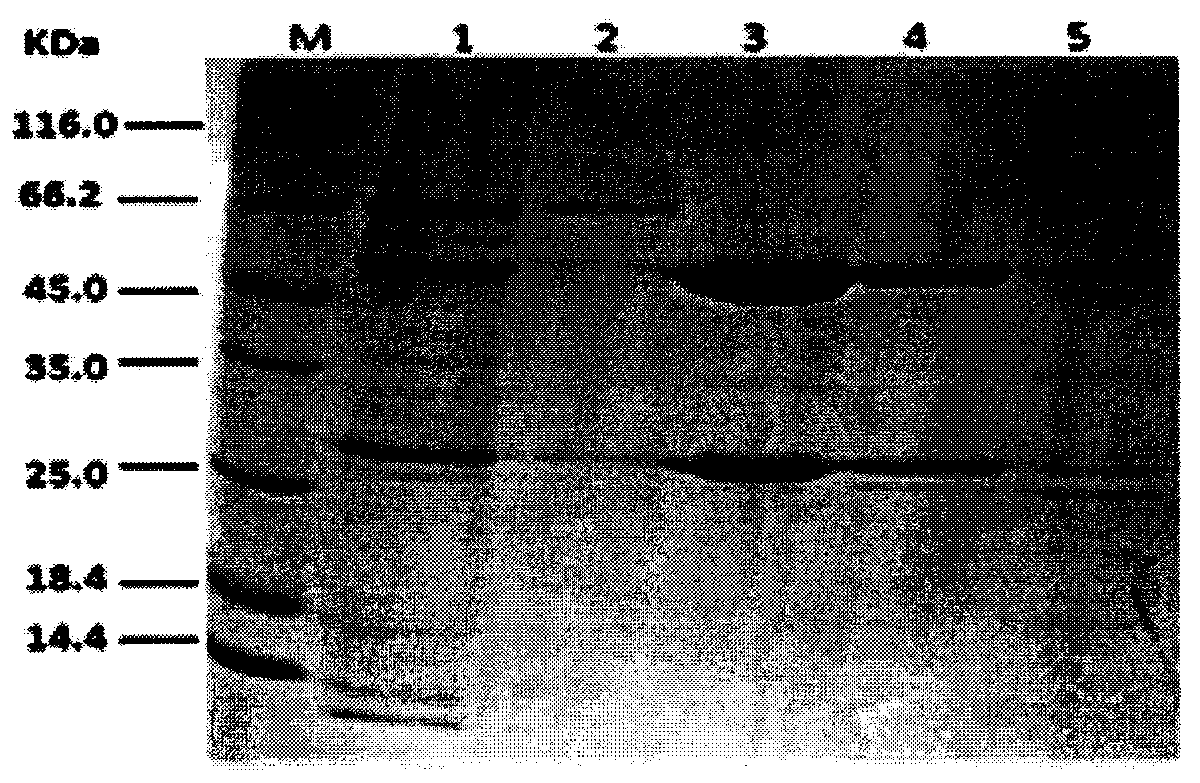
[0035] Affinity purification material column C1-2 purification of monoclonal antibody drugs cultured in CHO cells:
[0036] Prepare 10 mM HAc buffer as a balance solution, adjust pH 5.0 with 1N NaOH, prepare 100 mM Gly-HCl buffers with pH 4.5, pH 4.0 and pH 3.0.
[0037] Take a 1ml prepacked column of affinity purification material and connect it to AKTA, equilibrate to the baseline level with 10Mm PBS (150Mm NaCl, pH7.4) at a flow rate of 1ml / min, and prepare for loading.
[0038] CHO cell culture supernatant (4000rpm, centrifuged for 5in and discarded pellet), centrifuged at 3000g for 10min, took the supernatant and diluted it 10 times before loading the sample, washed with 10mM buffer solution at pH5.0, the flow rate was 1ml / min, To the baseline level, use 100mM Gly-HCl of pH 4.5, pH 4.0 and pH 3.0 to elute and collect the eluted products;
[0039] SDS-PAGE (12% SDS-PAGE gel) was used to detect the eluted sample, and the BCA kit was used to measure the protein concentratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com