Cryopreservation system with controlled dendritic freezing front velocity and cryopreservation method
A cryopreservation and low-temperature technology, which is applied in the field of cryopreservation of biopharmaceutical materials, can solve problems that are not conducive to yield and time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
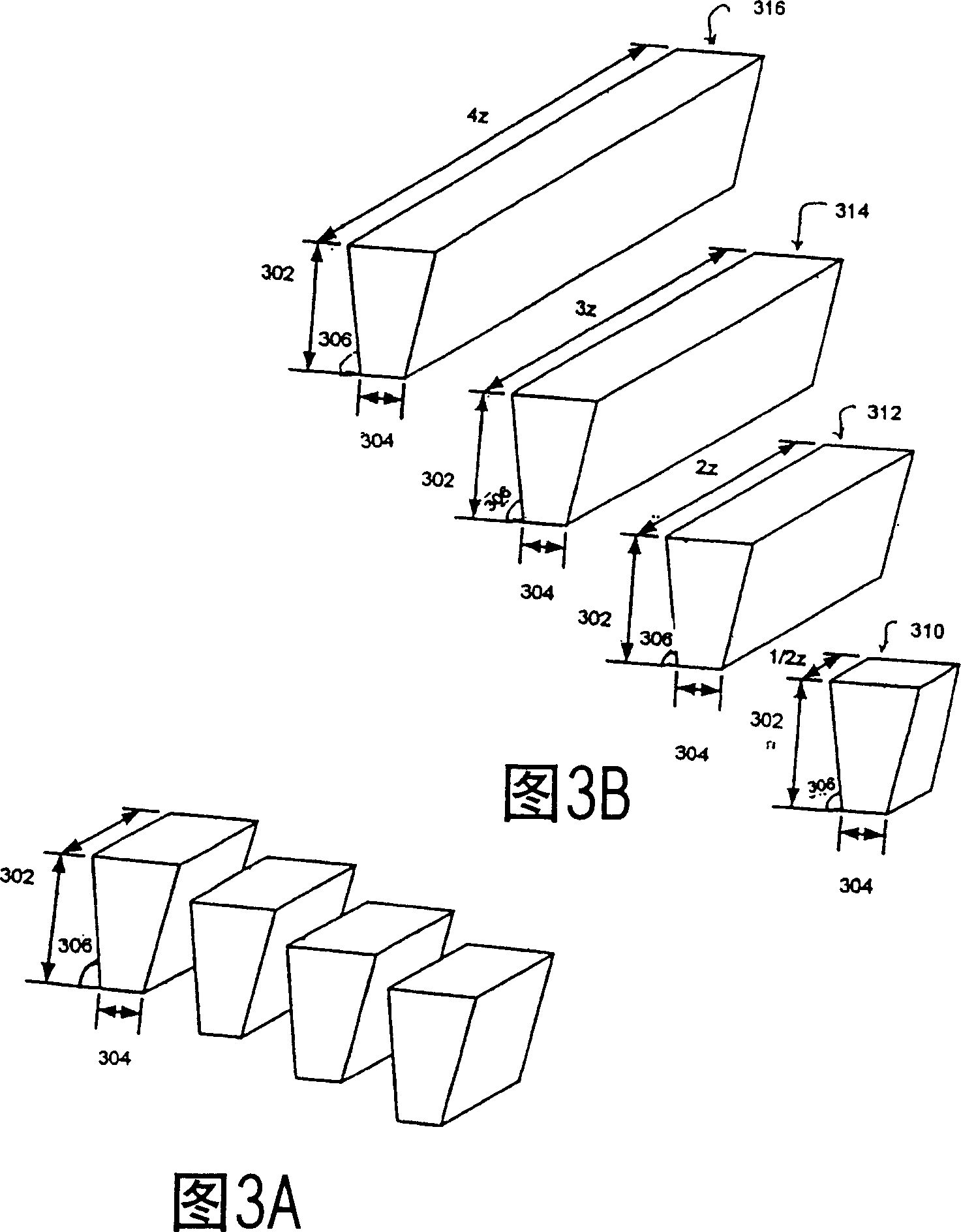
[0081] Example 1: The following example describes scaling using the thickness method. Both length and thickness are constant, 12cm and 40cm respectively. A 40-fold increase in volume can be obtained by freezing 10 cm or 42 cm long containers configured in parallel. Freezing time and freezing rate are kept constant.
[0082] growth factor
1
4
20
40
Volume (L)
5
20
100
200
Container length (cm)
10
42
20
417
The number of the 42cm length container
0.25
1
5
10
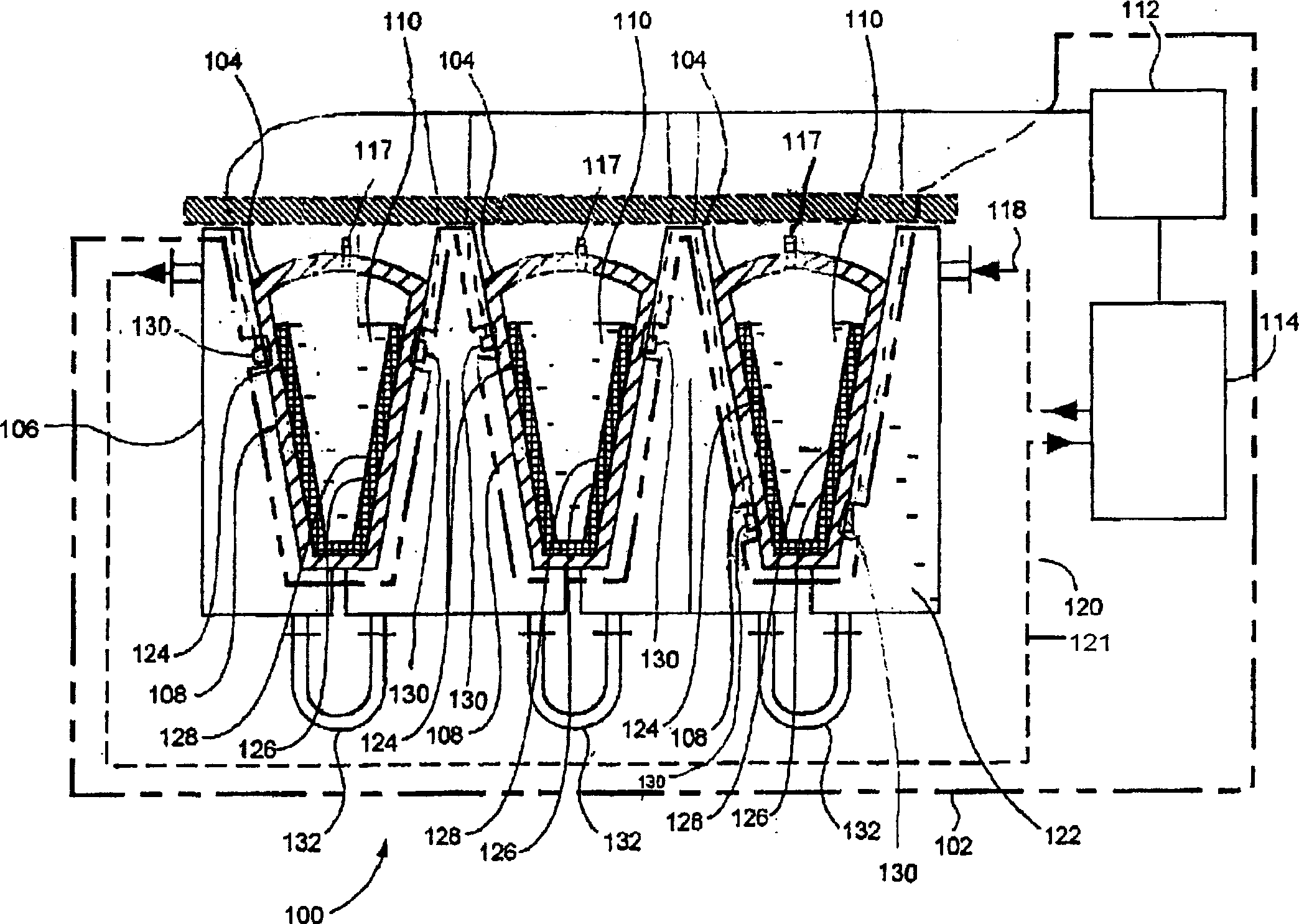
[0083] Figure 4 A biopharmaceutical material cryopreservation system 200 according to the present invention is shown that is substantially the same as the biopharmaceutical material cryopreservation system 100 except that the temperature sensor 130 is replaced by an internal temperature channel 230 and an internal temperature sensor 216 .
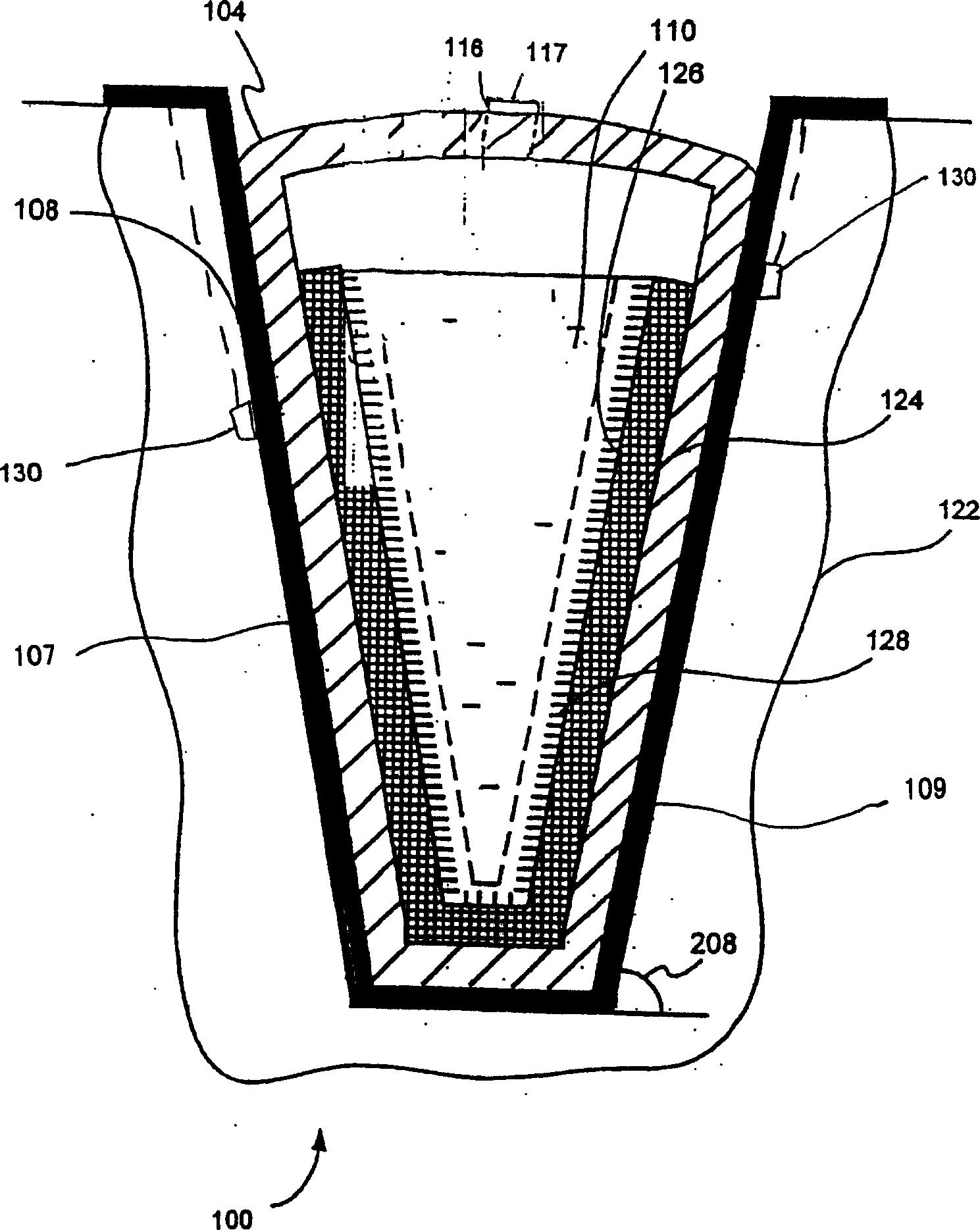
[0084] Figure 5 A portion of a biopharmaceutic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com