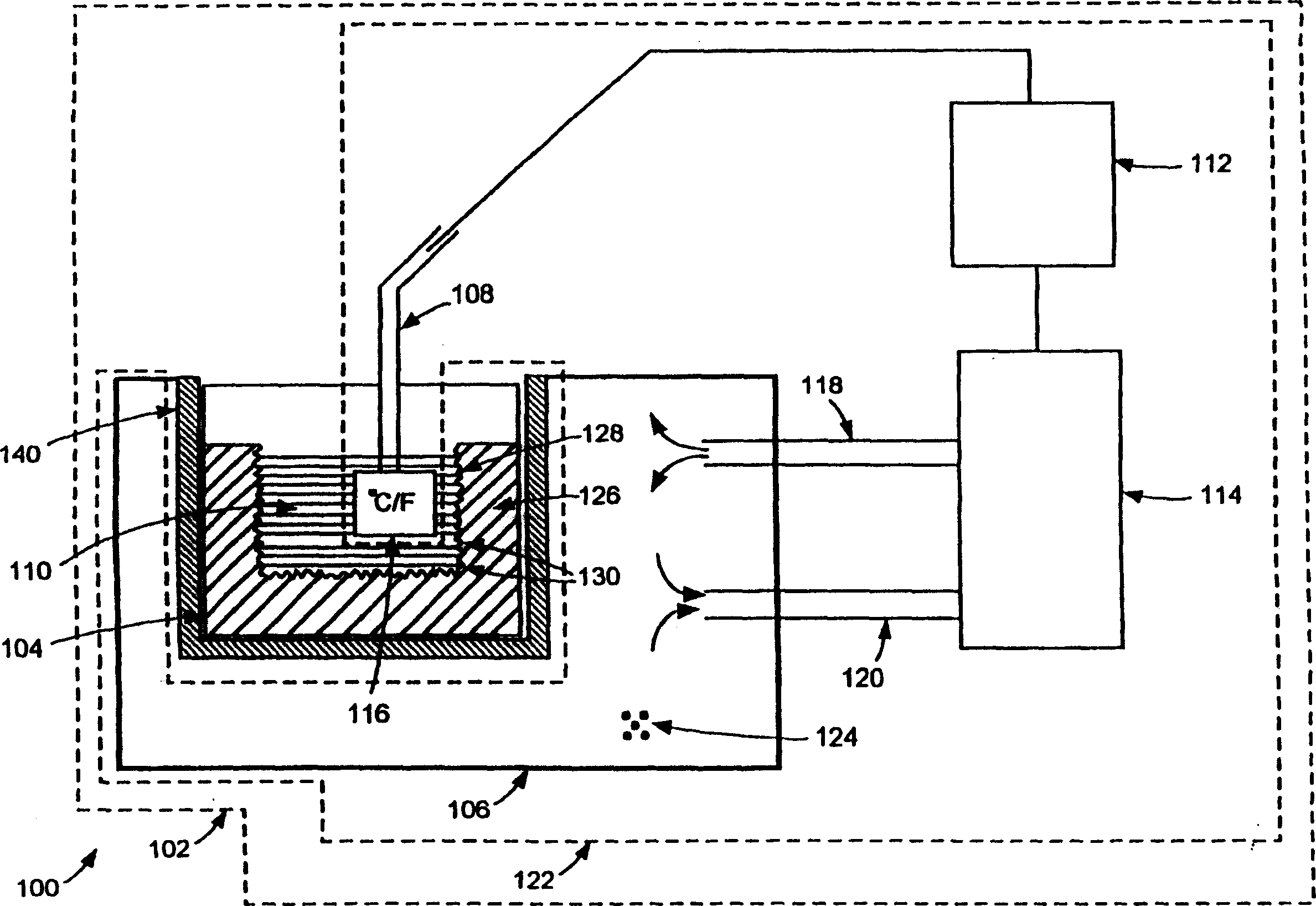
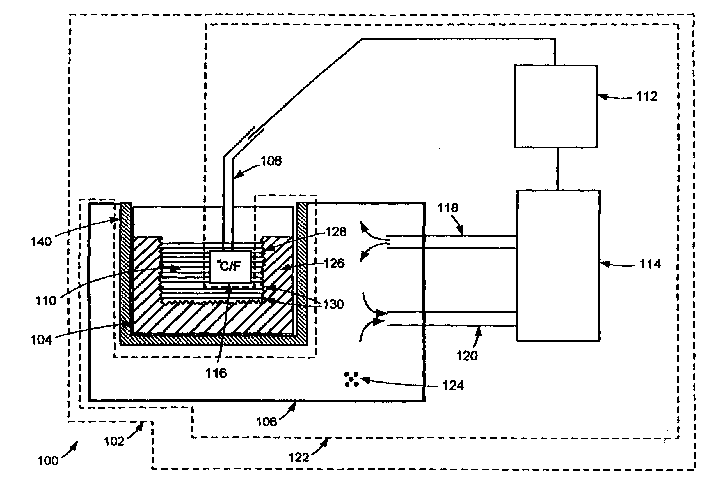
Cryopreservation system with controlled dendritic freezing front velocity
A technology of cryopreservation and freezing system, which is applied in the field of cryopreservation of biopharmaceutical materials, and can solve the problems of destroying sterility, container damage, loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The present inventors have surprisingly found that controlling the freezing rate in cryopreservation and cryogenic processing of biopharmaceutical materials can solve the aforementioned problems. According to one aspect of the present invention, the aforementioned problems can be partially or completely eliminated by ensuring that the cryopreservation and cryoprocessing of the biopharmaceutical material is carried out in a controlled manner such that the freezing rate of the biopharmaceutical material remains within a desired range .
[0014] When handling biopharmaceutical materials such as cells for cryopreservation, intracellular ice crystals can develop in the cells if they are frozen too quickly and with too high a water content. As a result, cells may rupture and / or lose their vitality. On the other hand, if cells are frozen too slowly, the cells are exposed to concentrated solutes for an extended period of time, which can lead to cellular damage. As another exa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com