Cryopreservation system with controlled dendritic freezing front velocity and method thereof
A technology of cryopreservation and freezing system, applied in the field of cryopreservation of biopharmaceutical materials, can solve problems such as integrity damage, contamination and loss of biopharmaceutical products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
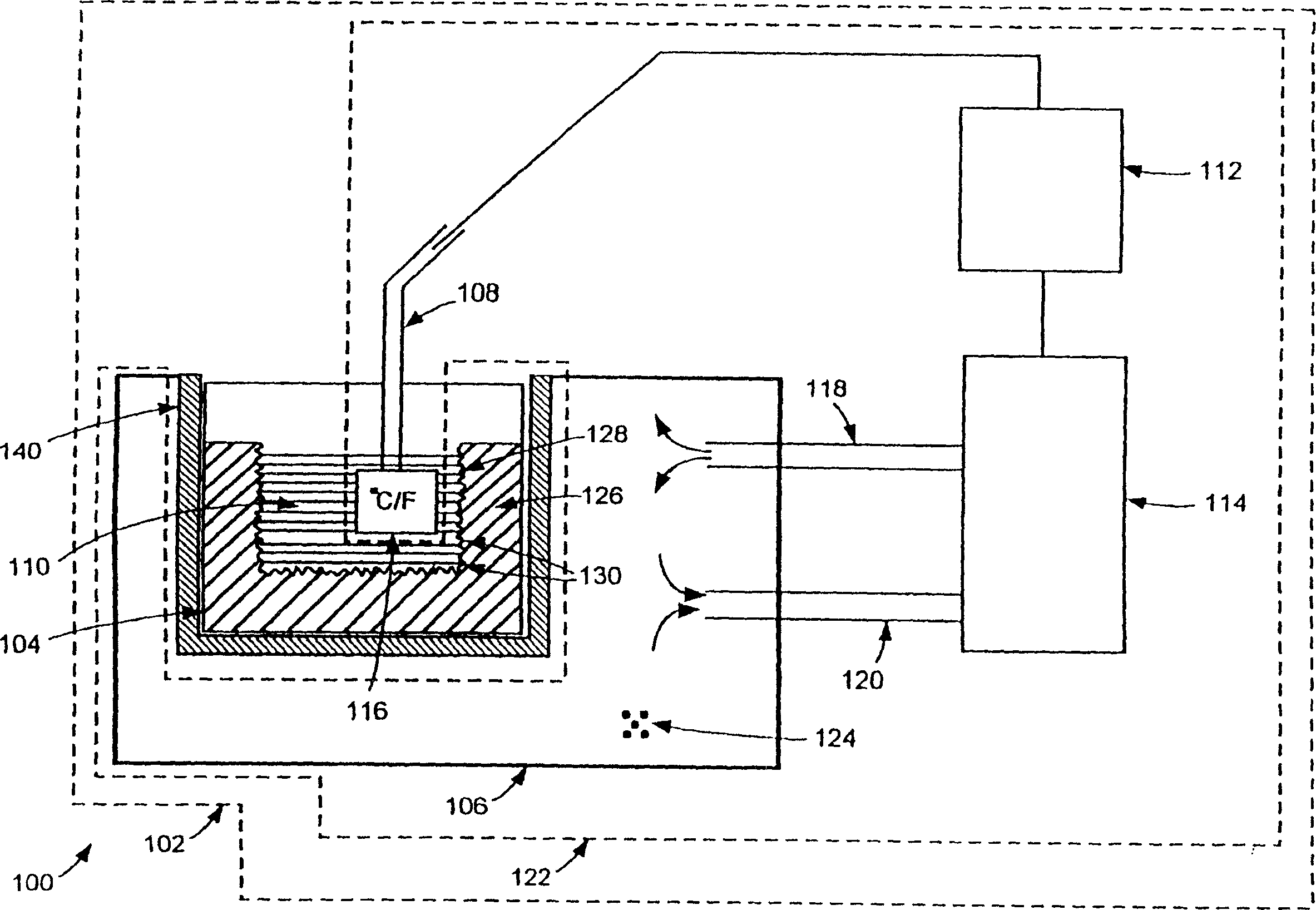
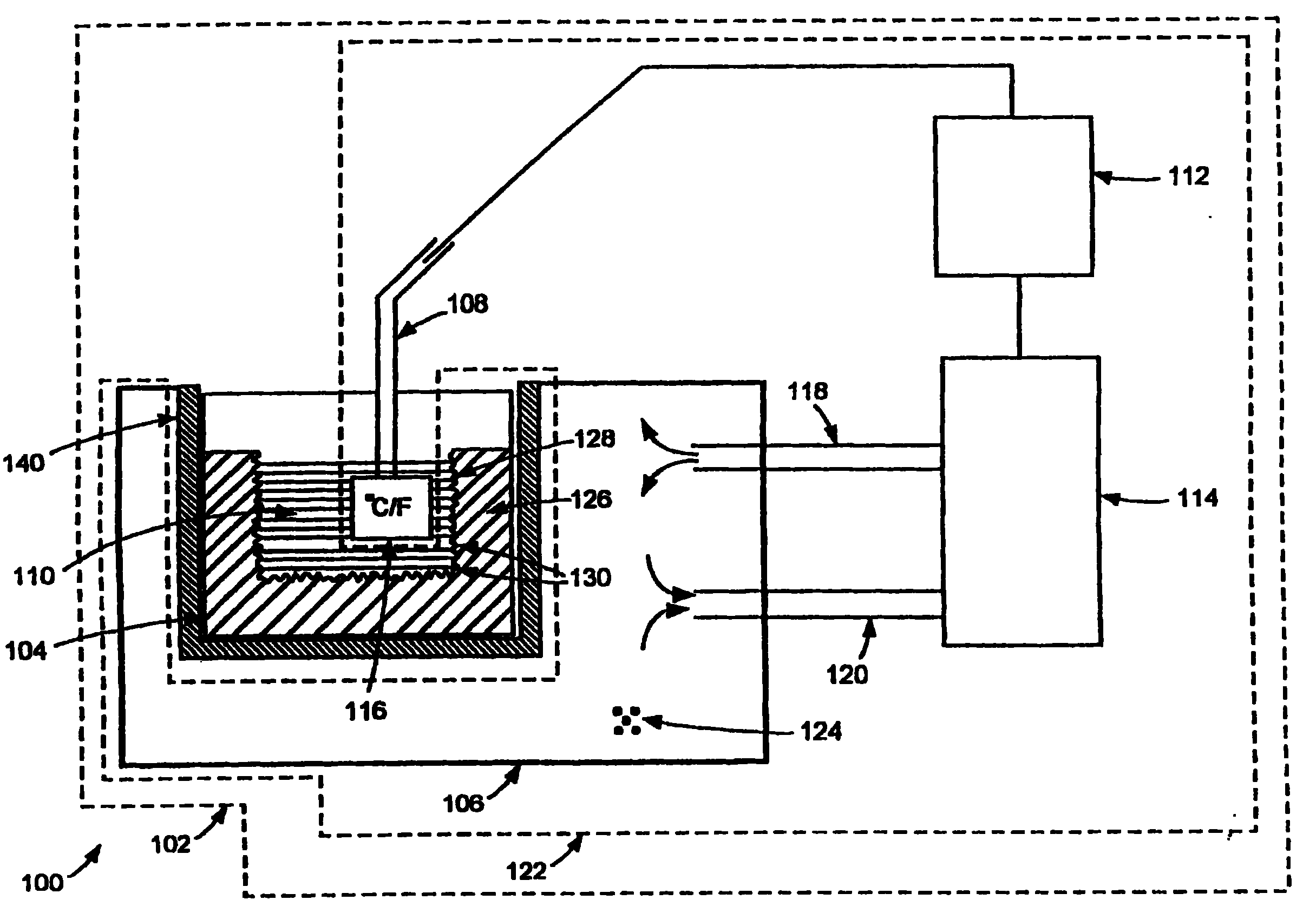
[0012] The inventors have unexpectedly found that controlling the freezing rate in cryopreservation and cryogenic processing of biopharmaceutical materials can solve the aforementioned problems. According to one aspect of the present invention, the aforementioned problems can be partially or completely eliminated by ensuring that cryopreservation and cryogenic processing of the biopharmaceutical material is performed in a controlled manner so that the freezing rate of the biopharmaceutical material is maintained within a desired range .
[0013] When processing biopharmaceutical materials such as cells for cryopreservation, intracellular ice crystals can form in the cells if the cells freeze too quickly and the water content is too high. As a result, cells may rupture and / or lose viability. On the other hand, if the cells are frozen too slowly, the cells are exposed to concentrated solutes for too long, potentially causing cell damage. As another example, freezing rate is al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com