Preventive and therapeutic agents for eye diseases
A therapeutic and pharmaceutical technology, applied in the field of eye disease prevention and therapeutic drugs, can solve problems that have no practical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
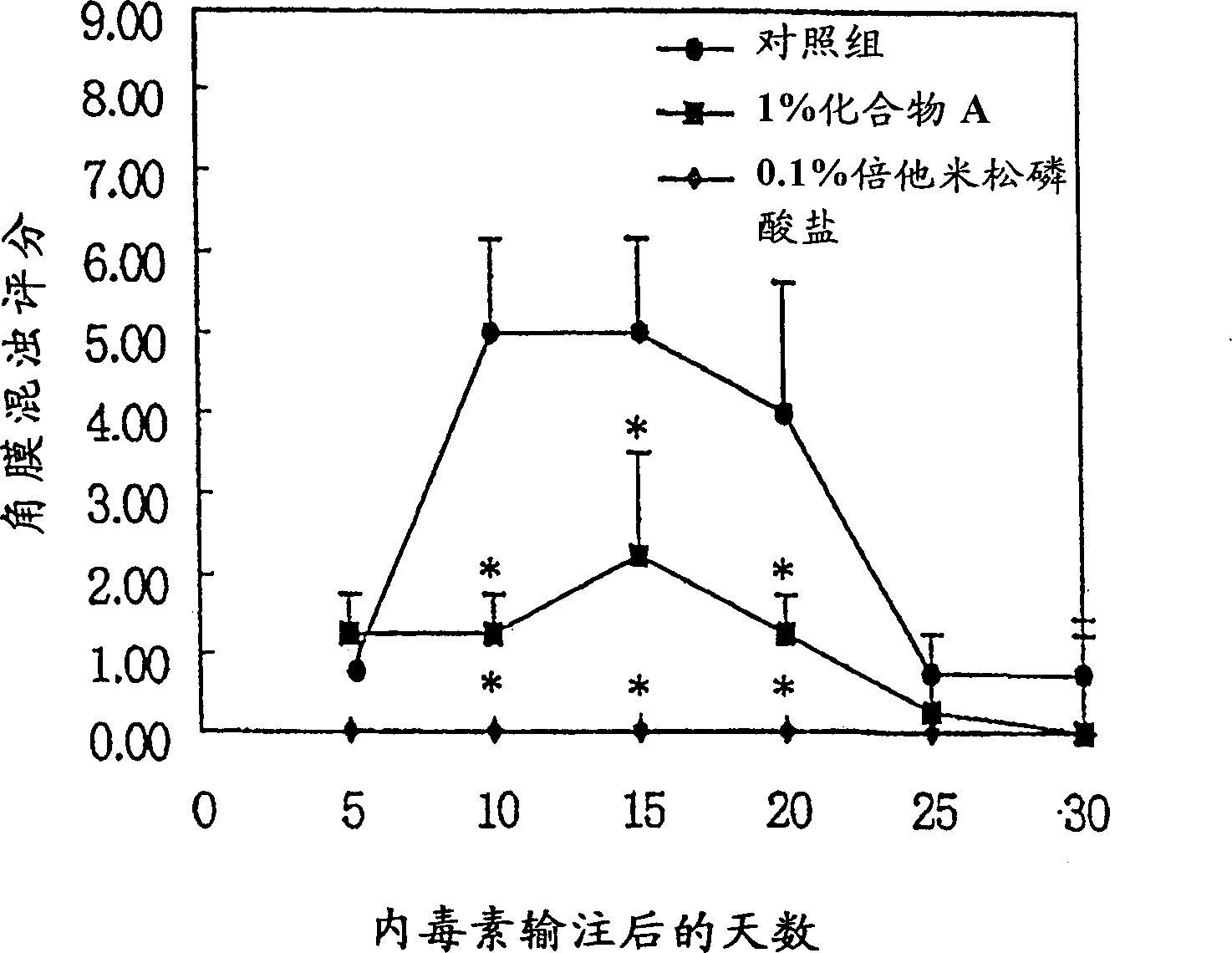
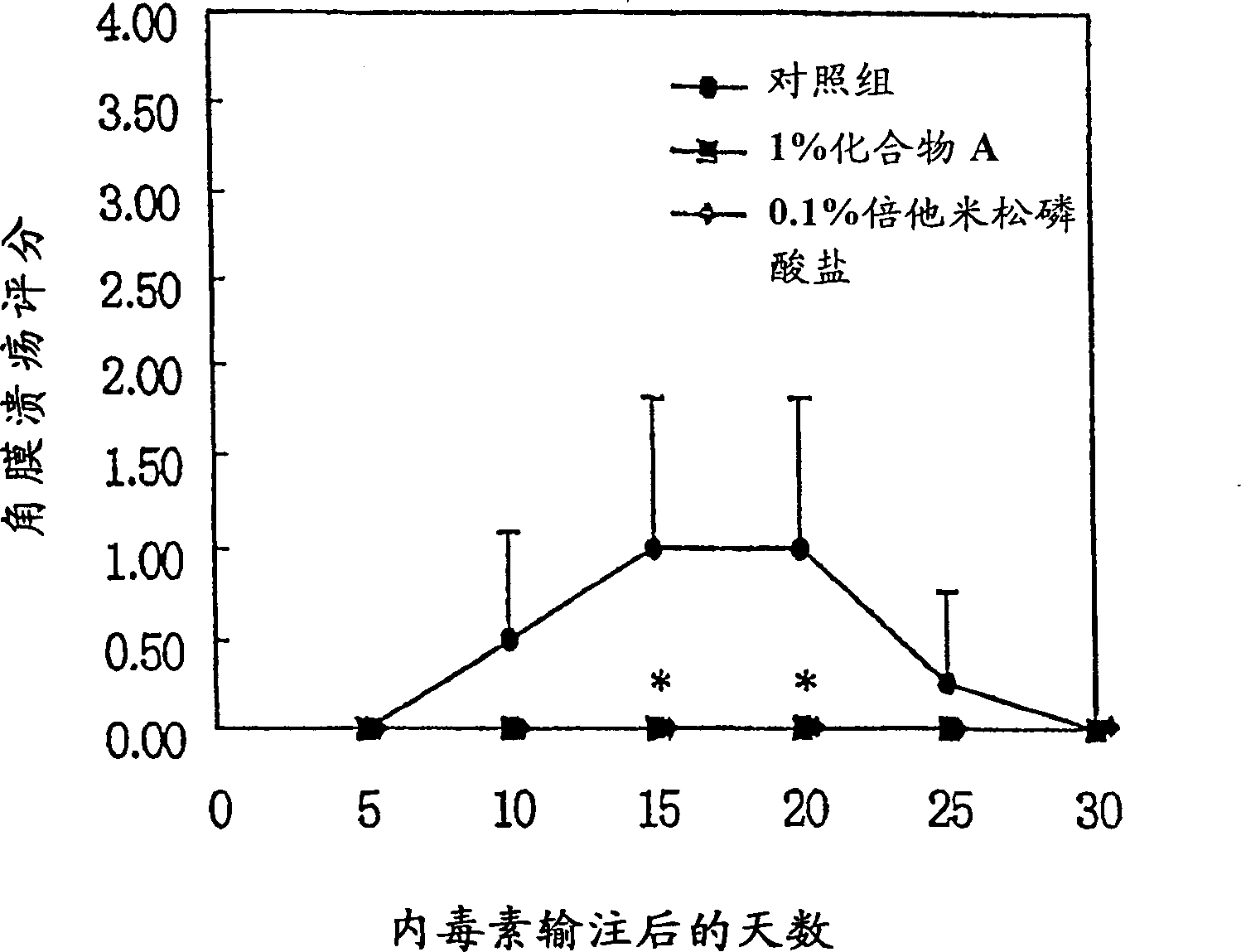
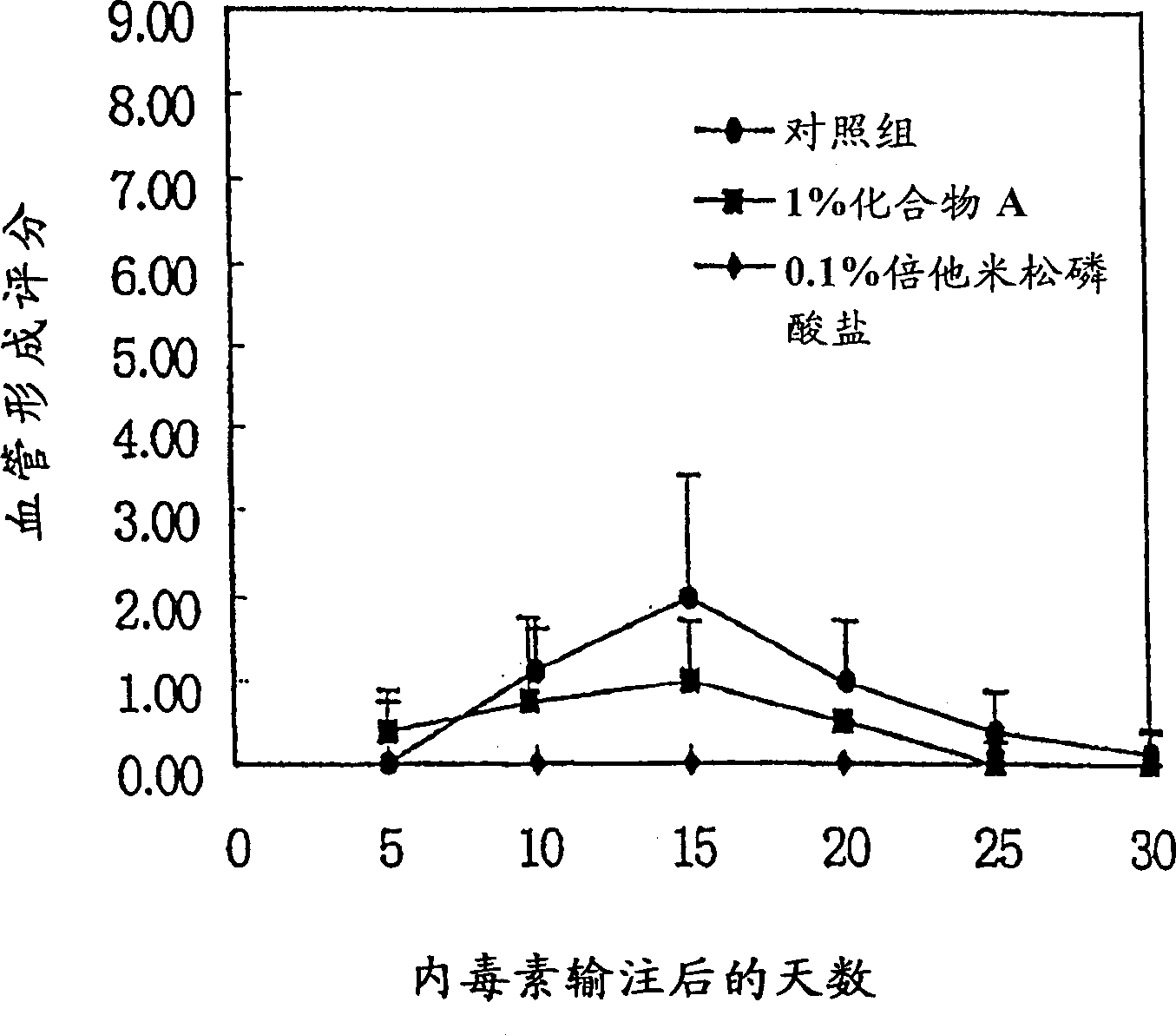
Image
Examples
Embodiment 1
[0080] Aqueous eye drops were prepared using the following composition. Ingredient Quantity Compound A 0.1g sodium chloride 0.9g sodium acetate 0.1g benzalkonium chloride 0.005g hydrochloric acid appropriate amount of sodium hydroxide appropriate amount of sterilized purified water to 100ml (pH6.0)
[0081] Compound A, sodium chloride, sodium acetate and benzalkonium chloride were dissolved in about 80 ml of purified water. The pH of the solution was adjusted to 6.0 with hydrochloric acid and sodium hydroxide. Sterilized purified water was added to make the total volume 100 ml, whereby an aqueous eye drop was obtained.
Embodiment 2
[0083] Eye drops of aqueous suspension were prepared using the following composition. Ingredient Quantity Compound A 1.0g sodium chloride 0.9g sodium acetate 0.1g polysorbate 80 0.2g benzalkonium chloride 0.005g hydrochloric acid appropriate amount of sodium hydroxide appropriate amount of sterilized purified water to 100ml (pH5.0)
[0084] Sodium chloride, sodium acetate, polysorbate 80, and benzalkonium chloride were dissolved in about 80 ml of purified water. The pH of the solution was adjusted to 5.0 with hydrochloric acid and sodium hydroxide, and then compound A was added and uniformly suspended using a homogenizer. Sterilized purified water was added to make the total volume 100 ml, whereby an aqueous suspension eye drop was obtained.
Embodiment 3
[0086] Eye drops of aqueous suspension were prepared using the following composition. Ingredient amount Compound A 0.5g concentrated glycerin 2.6g Sodium acetate 0.1g Hydroxypropyl methylcellulose 0.2g Methyl p-hydroxybenzoate 0.03g Propyl p-hydroxybenzoate 0.02g Hydrochloric acid Appropriate amount Sodium hydroxide Appropriate amount Sterilization and purification Water to 100ml (pH5.0)
[0087] Dissolve methylparaben and propylparaben in warm about 80 ml of purified water. In this solution, hydroxypropylmethylcellulose was dispersed, and then cooled to room temperature to be dissolved. Concentrated glycerin and sodium acetate were added to the solution, and the pH was adjusted to 5.0 with hydrochloric acid and sodium hydroxide. Compound A was added to the solution and uniformly suspended using a homogenizer. Sterilized purified water was added to make the total volume 100 ml, thereby obtaining eye drops as an aqueous suspension. Example 4
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap