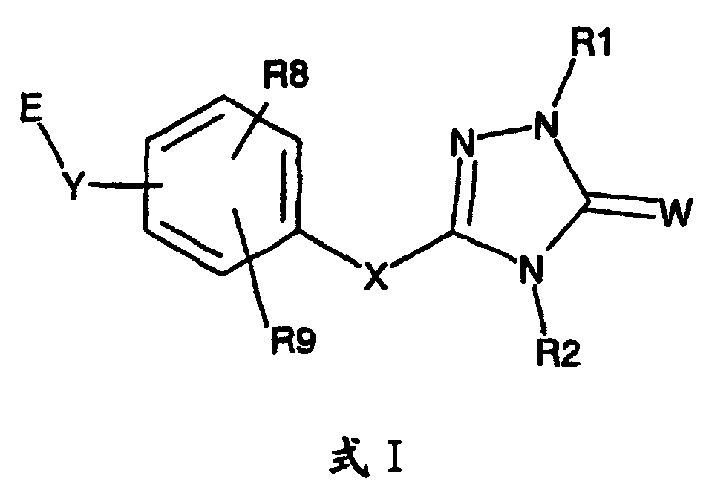
Pexoxisome proliferator activated receptor alpha agonists
一种化合物、溶剂化物的技术,应用在过氧化物酶体增殖子激活受体α激动剂领域,能够解决不能降低甘油三酯和LDL-胆固醇水平、体重增加、增加HDL-胆固醇水平等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
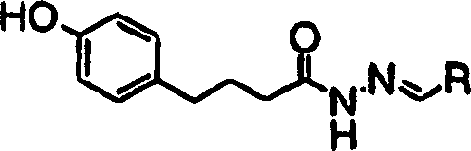
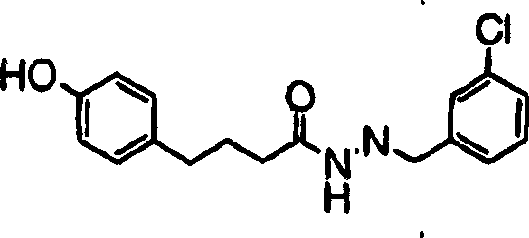
[0221] Compound 1(1)
[0222] Compound 1 shown below was prepared according to the following steps: Step A: Preparation:
[0223] To a suspension of 4-(4-hydroxyphenyl)butanylhydrazide (0.5 g, 2.58 mmol) in isopropanol (5 mL) was added 3-chlorobenzaldehyde (Aldrich, 420 mg, 3 mmol) followed by p-toluene Sulfonic acid (25 mg). The reaction was stirred at room temperature for 20 hours. A solid precipitated out which was filtered, washed with isopropanol (0.25 mL) and dried to give the product as a solid. MS: m / z (M + +1): 317
[0224] R
MS: m / z (M + +1)
3-Methylphenyl
297
Phenyl
283
2,4-Difluorophenyl
319
2-Methylphenyl
297
3-methoxyphenyl
313
[0225] To a solution of the product from Step A (650 mg, 2.05 mmol, Example 1) in 5 mL of a mixture of isopropanol, tetrahydrofuran and acetic acid (1:1:0.3) was added sodium cyanoborohydride (1.25 g, 20 mmol)....
Embodiment 2
[0236] Compound 2(1)
[0237] The compound represented by the following formula was prepared as follows: Step A: Preparation:
[0238] Add boron tribromide (50 g, 200 mmol) to CH in CH over 1 hour 2 Cl 2 To a cold (0°C) solution in (50 mL) was added dropwise methyl 4-(4-methoxyphenyl)butanoate (15.5 g, 74.4 mmol) in CH 2 Cl 2 (100mL). After continuing to stir at 0 °C for 1 hour, the reaction mixture was washed with 1:1 CH 3 OH:CH 2 Cl 2 (120 mL) and stirred overnight at room temperature. The mixture was concentrated to give an oil which was partitioned between ethyl acetate (150 mL) and water (150 mL). The aqueous layer was extracted with ethyl acetate (2×50 mL), and the combined organic extracts were washed with water (50 mL), brine (50 mL), and dried (Na 2 SO 4 ), followed by concentration to obtain the desired phenol as an oil. C 11 h 14 o 3 (MW=194.23); MS: m / z (M + +1)=195 Step B: Preparation:
[0239] The phenol (18.6 g, 96 mmo...
Embodiment 3
[0248] Compound 3(1) Step A: Preparation:
[0249] To a solution of the methyl ester—compound 2(1) (100 mg, 0.29 mmol) in DMF (2 mL) was added 3,4,5-trimethoxybenzyl chloride (129 mg, 0.6 mmol) and potassium carbonate powder (350 mg , 2.53 mmol), and the resulting mixture was heated at 45°C for 24 hours. After cooling to room temperature, the reaction mixture was diluted with water (10 mL) and extracted with ethyl acetate (3 x 3 mL). The combined organic extracts were concentrated to an oil and purified by flash chromatography (gradient elution, 1:4 EtOAc:Hexane - 4:1 EtOAc:Hexane) to afford the desired product as an oil thing. C 27 h 35 N 3 o 7 (MW=513.60); MS: m / z (M + +1)=514
[0250] R
MS: m / z (M + +1)
3,5-dimethoxyphenyl
484
4-biphenyl
500
3-Chlorophenyl
458
3-Chloro-4-methylphenyl
472
Bis-trifluoromethylphenyl
560
3,4-Difluorophenyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com