Pharmaceutical preparation
A preparation and mucosal drug delivery technology, applied in the field of pharmaceutical preparations of enteric polymers, can solve the problems of reduced bioavailability of bicalutamide, increased systemic exposure, and limited maximum systemic exposure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
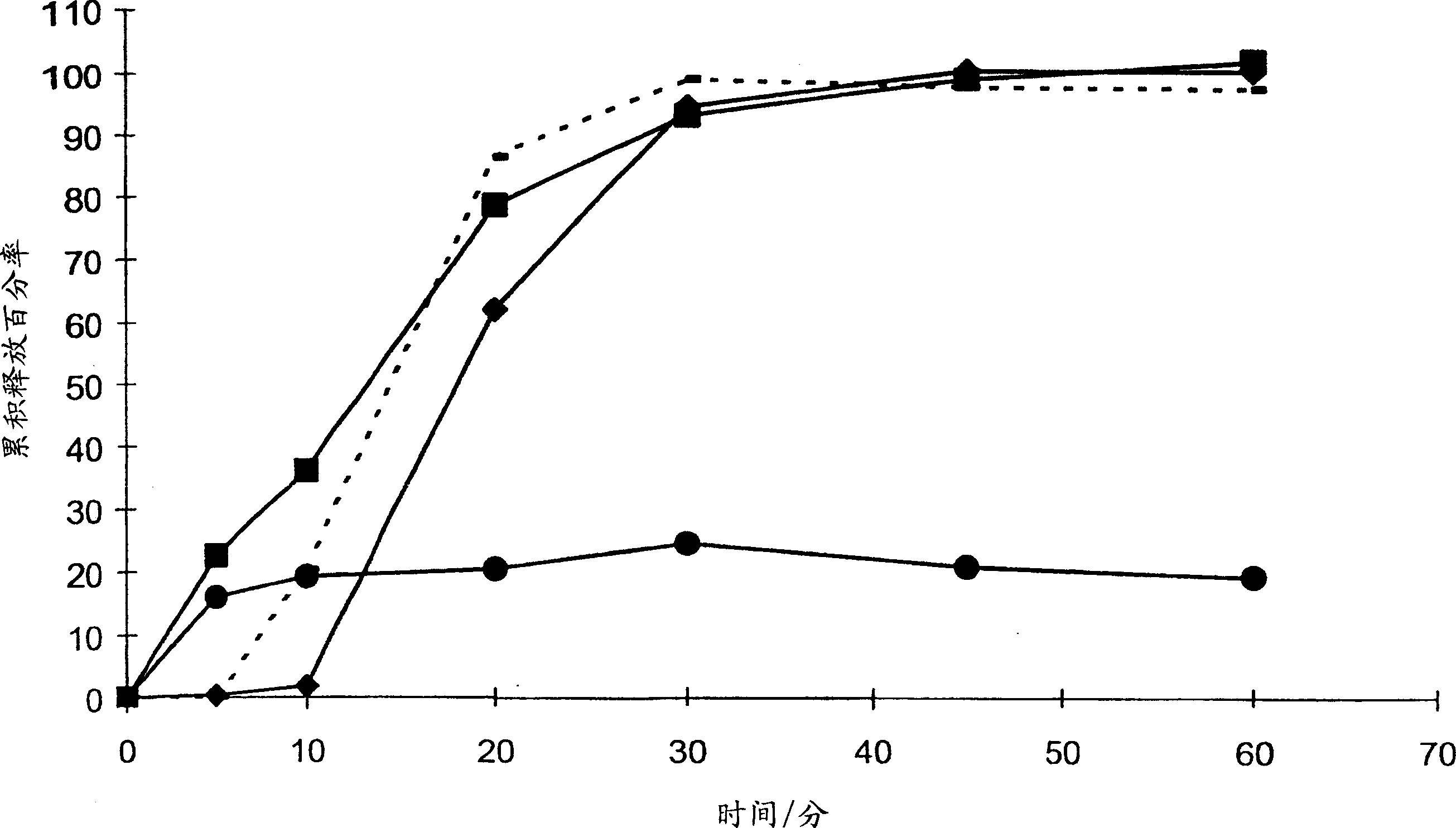
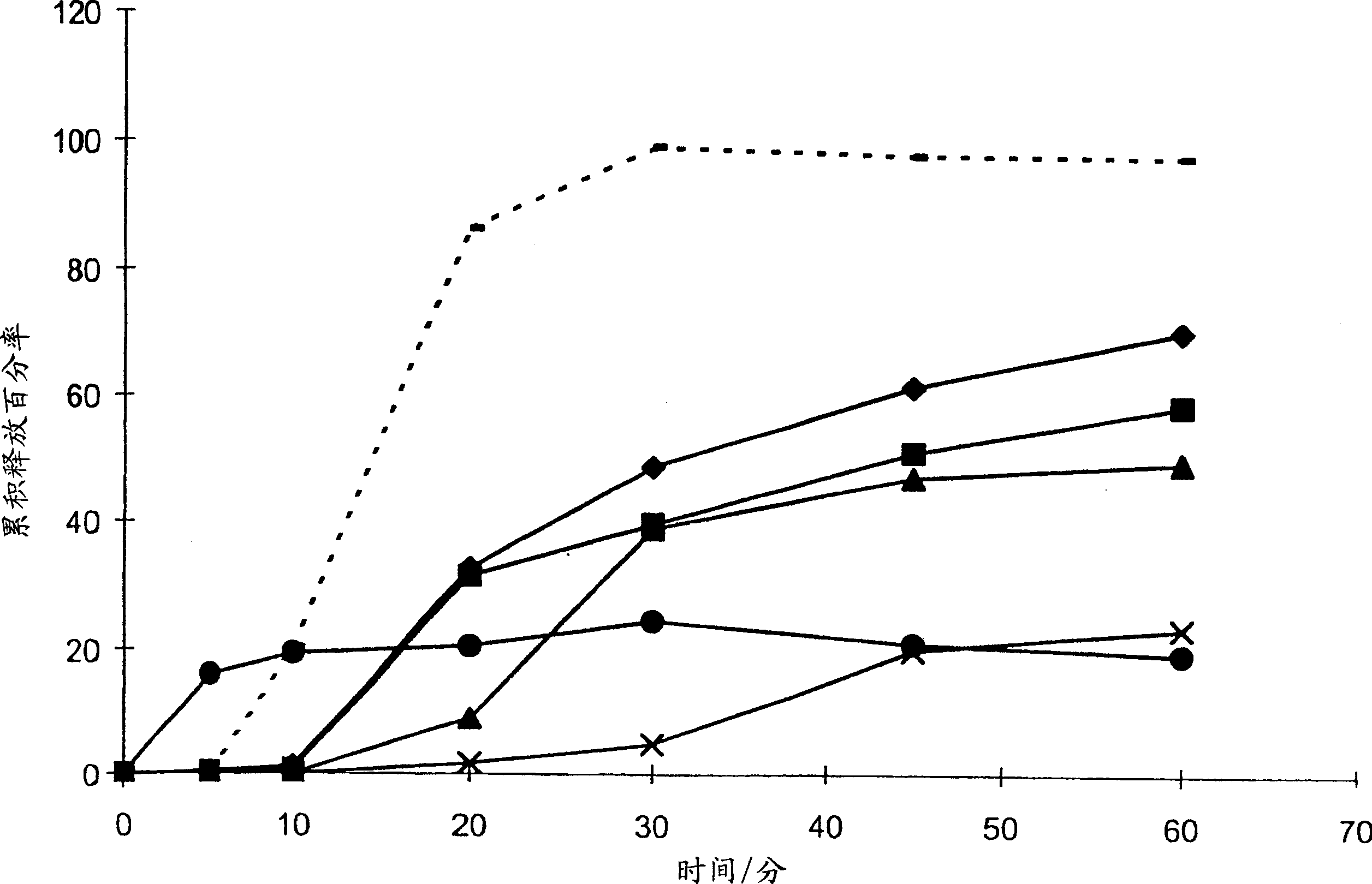
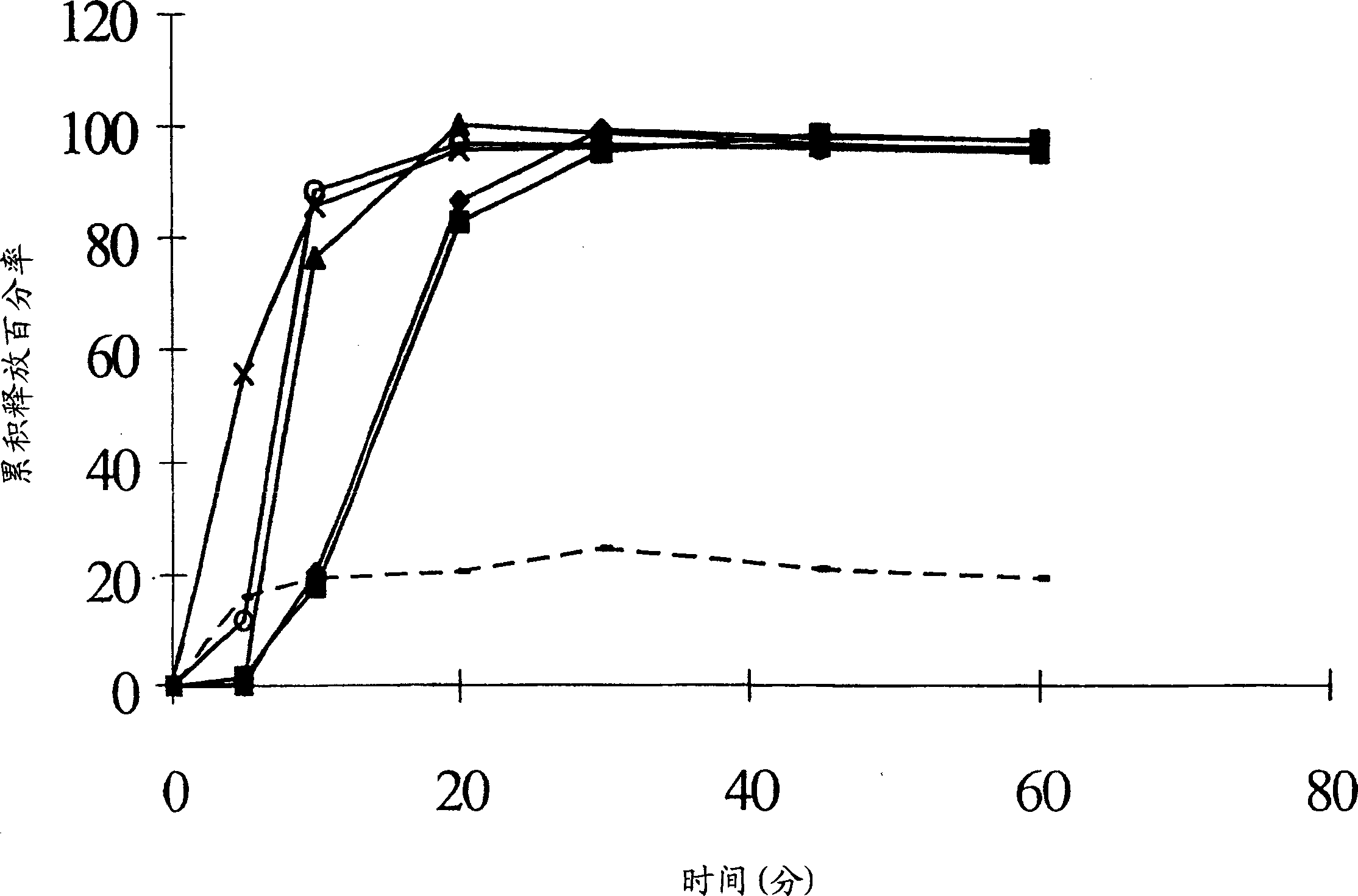
[0046] The inventors chose to investigate solid dispersions as a possible means of increasing the efficacy of bicalutamide. The aim is to improve efficacy by increasing the bioavailability of bicalutamide or reducing interpatient variability in bicalutamide plasma concentrations, or both.
[0047] In order to increase drug bioavailability in general, the prior art teaches various possible polymers for solid dispersions. The present inventors have now surprisingly found that the efficacy of bicalutamide can be enhanced by specifically formulating bicalutamide with an enteric polymer having a pKa of 3 to 6 in a solid dispersion. As part of a non-limiting example, the following demonstrates that the use of other polymers does not bring about an increase in the efficacy of bicalutamide.
[0048] A variety of substances are routinely used to coat pharmaceutical tablets, capsules and granules, and the coated granules can be compressed into tablets or used to fill capsules. See Sch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com