Process for prepn. of racemic citalopram and/or S-or R-citalopram by separation of mixture of R-and S-citalopram
A technology for citalopram and its mixture, applied in the field of preparing racemic citalopram and/or S- or R-citalopram by separating R- and S-citalopram mixture, can solve the problem of not being able to meet the requirements of S-citalopram Citalopram specifications and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
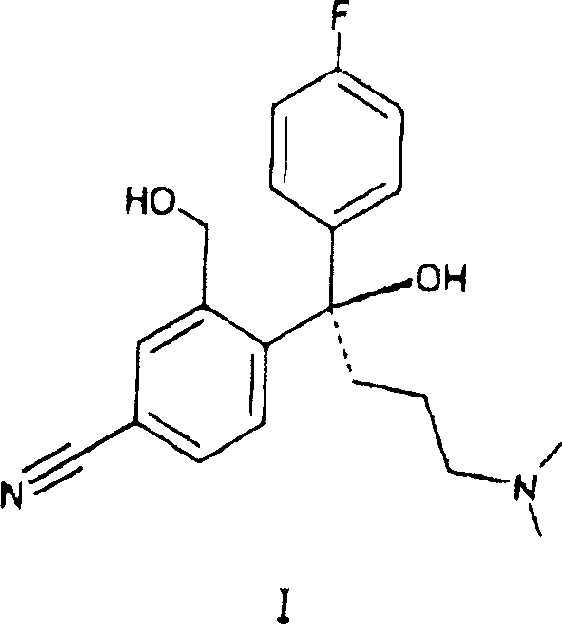
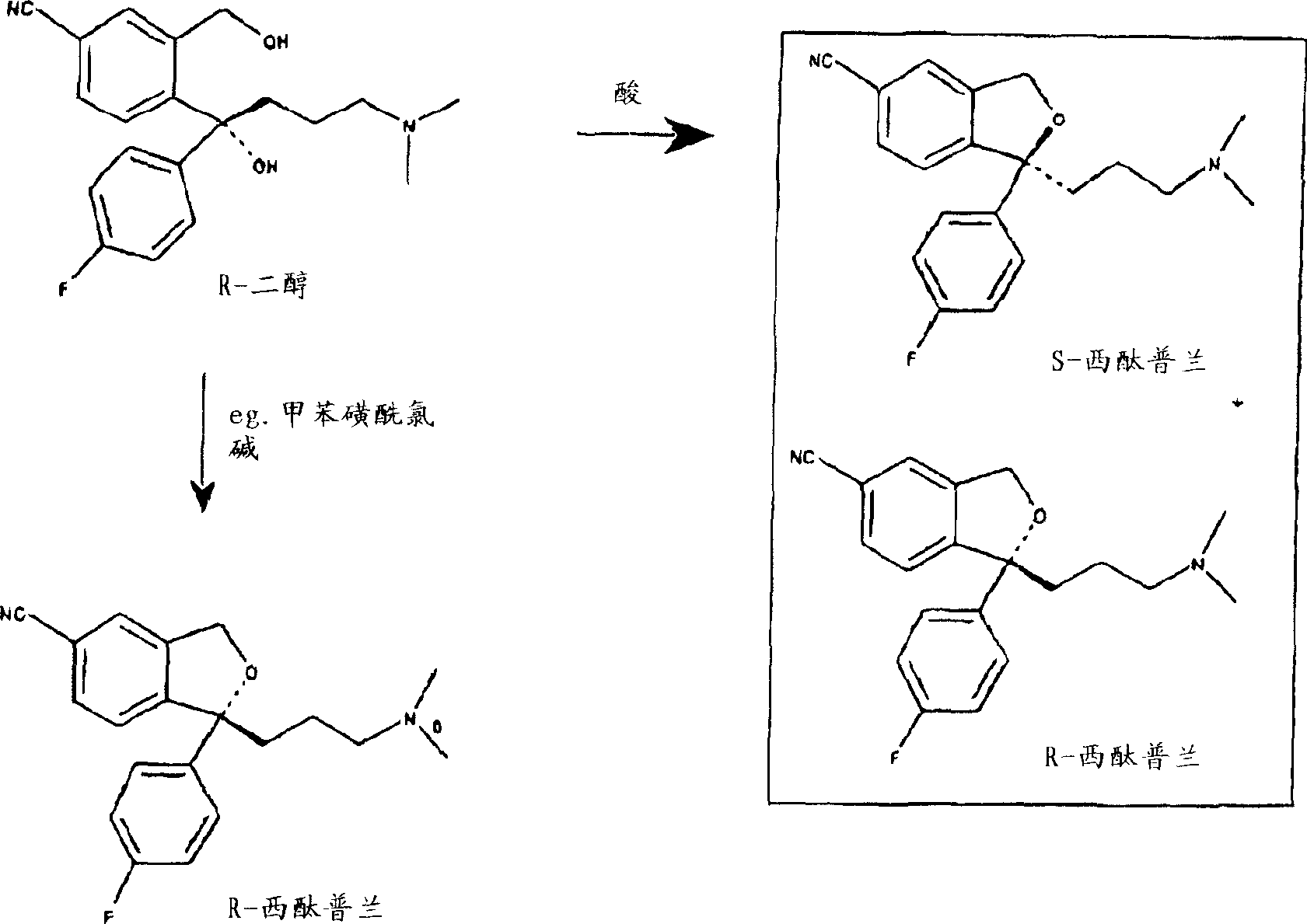
[0068] From R-4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)benzonitrile by reaction with different acids in acetonitrile (R / S=95.7 / 4.3) Preparation of Citalopram
[0069] general method
[0070] R-4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)benzonitrile (67.5g, R / S= 95.7 / 4.3) was dissolved in acetonitrile (37g), stirred at room temperature, added the mixture of acid and ice (or water) (the amount of acid and the amount of ice are listed in Table 1), and the reaction mixture was stirred at 78-85°C (reaction time Listed in Table 1), cooling the reaction mixture, adding water and toluene (315ml), then adding ammonia (25%, by weight), reaching pH9.5-10.5, the mixture was heated to 50-55 ° C (5-10 minutes), The phases were separated, toluene (50ml) was added to the aqueous phase, and stirred at 50-55°C (5-10 minutes). The phases were separated, the toluene phases were combined, washed 3 times with water (3 x 65 ml), and ...
Embodiment 2
[0074] From R-4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)benzonitrile by reaction with different acids in toluene (R / S=95.7 / 4.3) Preparation of Citalopram
[0075] general method
[0076] R-4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)benzonitrile (67.5g, R / S= 95.7 / 4.3) was dissolved in toluene (315ml), stirred at room temperature, added the mixture of acid and ice (or water) (the amount of acid and the amount of ice are listed in Table 2), and the reaction mixture was stirred at 78-85°C (reaction time Listed in Table 2), cooling the reaction mixture, adding water, and then adding ammonia (25%, by weight), to reach pH9.5-10.5, the mixture was heated to 50-55 ° C (5-10 minutes), phase separation, toluene The phase was washed 3 times with water (3 x 65 ml) and the toluene was removed under reduced pressure at up to 60°C to give the product as an oil.
[0077] Prepare citalopram acid by the above-mentioned general met...
Embodiment 3
[0080] From R-4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxyl Methyl)benzonitrile (R / S=95.7 / 4.3) to prepare citalopram HBr
[0081] acid cyclization
[0082] R-4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)benzonitrile (67.5g, R / S= 95.7 / 4.3) was dissolved in toluene (315ml), stirred at room temperature, added a mixture of sulfuric acid (26g, 96%) and ice (10g), stirred the mixture for 2 hours at 78-85°C, cooled the reaction mixture, added 40ml of water, and then Aqueous ammonia (25%, by weight) was added to reach pH 9.5-10.0, the mixture was heated to 55° C. (10 minutes), the phases were separated, the toluene phase was washed with water 3 times (3×65 ml), and the pressure was reduced at a maximum of 60° C. Removal of the toluene gave an oil (Oil A), yield 63 g (99%).
[0083] Basic Cyclization of Unstable Esters
[0084] R-4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)benzonitrile (33.7g, R / S= 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com