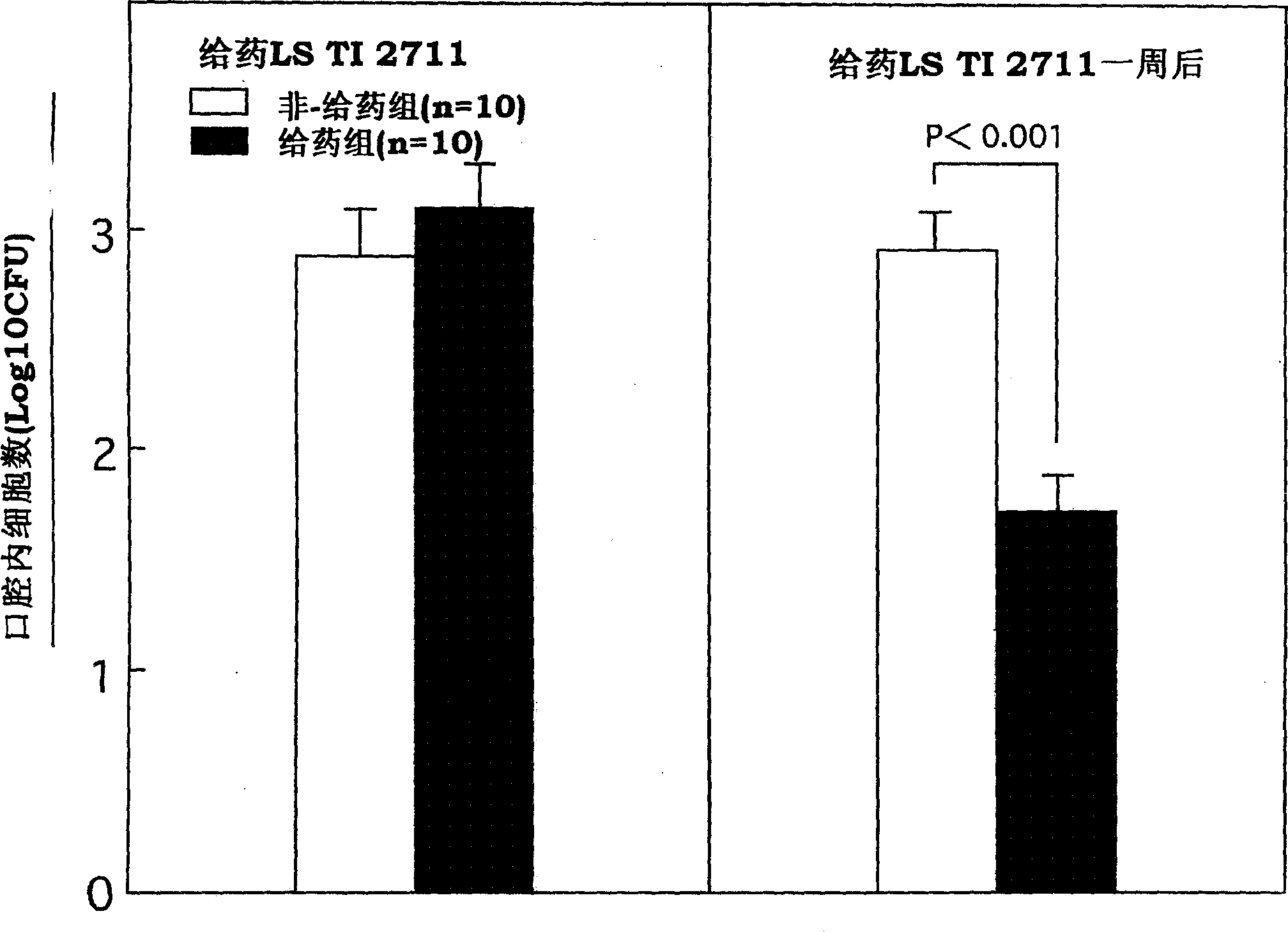
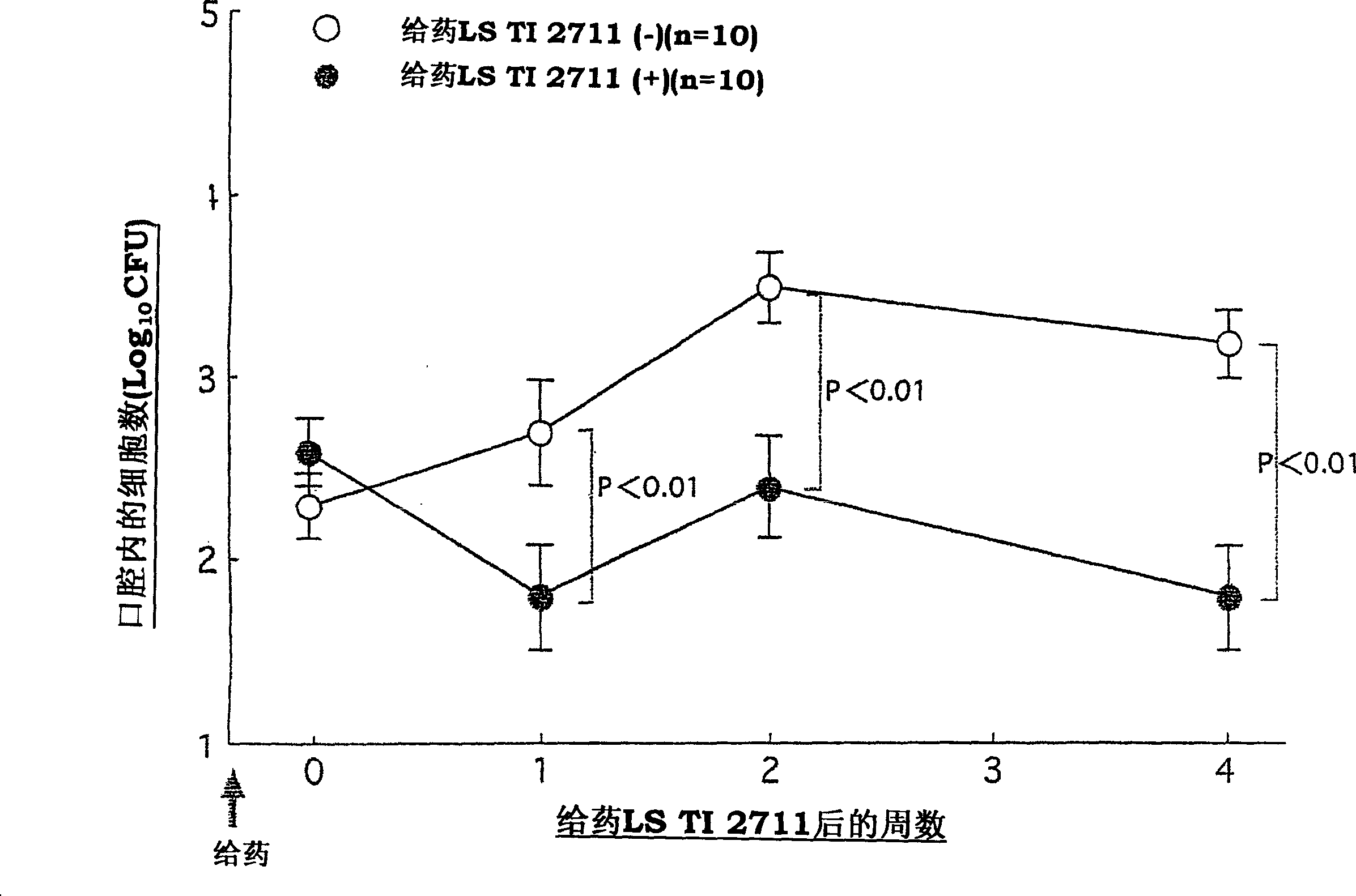
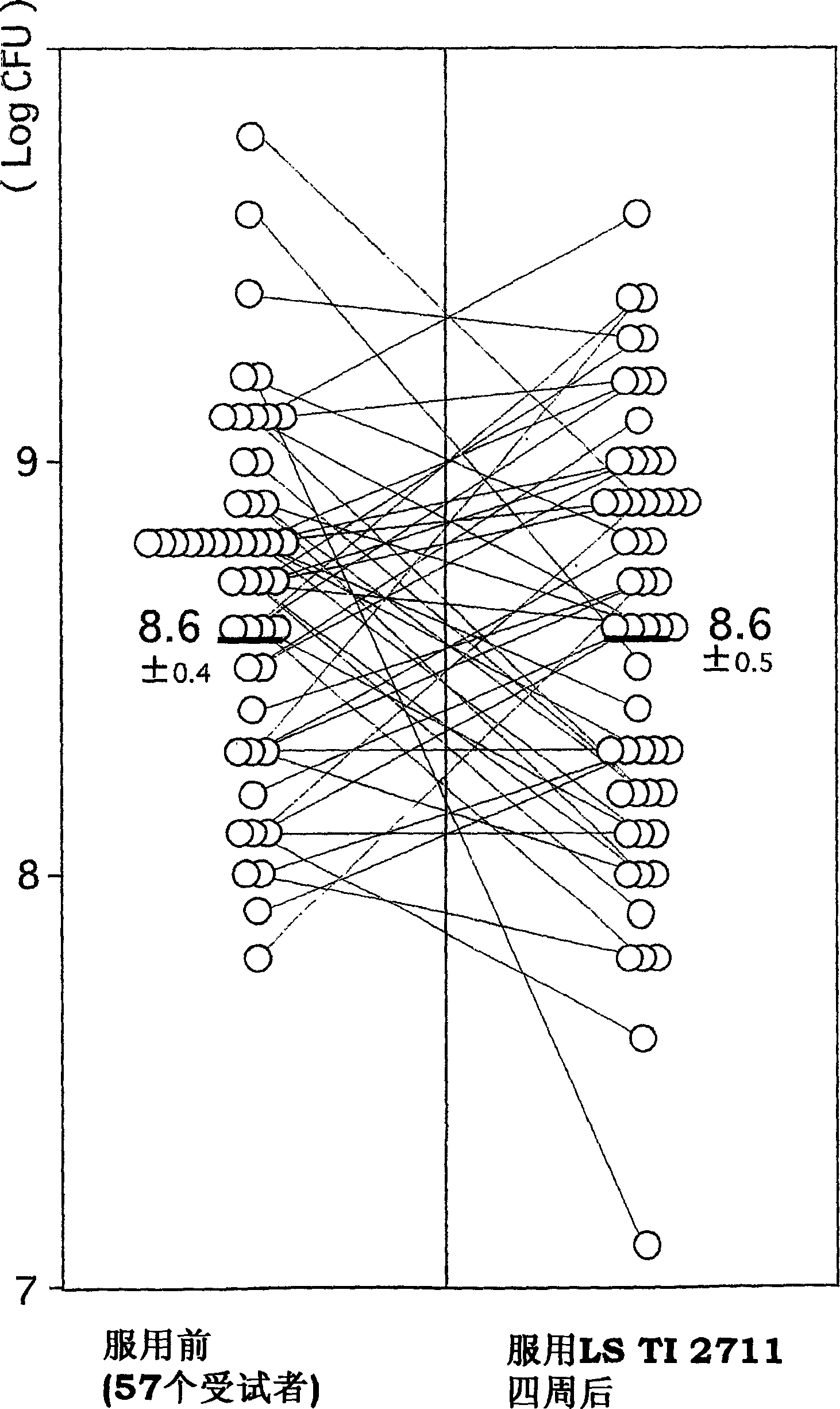
Vital cell preparations containing lactic acid bacterium as the ative ingredient and food containing lactic acid
一种乳酸杆菌、活性成分的技术,应用在含有效成分的医用配制品、乳杆菌、食品制备中使用的细菌等方向,能够解决没有公开消除或者抑制牙周疾病和龋齿的数据等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Example 1: Preparation of dried cells of Lactobacillus salivarius TI 2711 strain
[0166] Lactobacillus salivarius TI 2711 strain was inoculated into Briggs fresh broth containing 0.3% calcium carbonate, and statically cultured at 37°C for 18 hours. After the cultivation, the culture was centrifuged at 7,000 rpm for 15 minutes to obtain cells with a concentration of 1 / 100 volume.
[0167] Then, a dispersion medium containing 5% (weight) sodium glutamate, 5% (weight) soluble starch, 5% (weight) sucrose and concentrated cells were mixed in equal amounts, and the pH value of the mixture was adjusted to 7.0. Freeze at 40°C or lower and lyophilize. The obtained freeze-dried cells are passed through a 60-mesh sieve to prepare a powder to obtain the lactobacillus powder of the present invention. As for the preservation stability of the bacteria of the present invention, even when the Lactobacillus powder was stored at room temperature (24°C) for 10 months under closed conditions, ...
Embodiment 2
[0168] Example 2: Preparation of medicinal live bacterial preparations (tablets)
[0169] According to the 12th revision of the Japanese Pharmacopoeia guidelines, the general rules for pharmaceutical preparations, the provisions of "tablets", 2 grams of Lactobacillus salivarius TI 2711 strain dried cell powder prepared in Example 1 (cell number: 5× 109 CFU / g), 161 g of lactose (Japanese Pharmacopoeia), 116 g of starch (Japanese Pharmacopoeia), 20 g of binder polyvinylpyrrolidone (Japanese Pharmacopoeia) and 0.8 g of magnesium stearate (Japanese Pharmacopoeia) as a lubricant They were brought together and mixed uniformly, and the mixture was compressed into a mold with a tablet manufacturing equipment to obtain 290 grams of ordinary tablets (300 mg per tablet).
Embodiment 3
[0170] Example 3: Preparation of food containing Lactobacillus (sugar-flavored tablets)
[0171] With reference to the Japanese Pharmacopoeia Guidelines for the 12th revision, the general rules for pharmaceutical preparations and the provisions of "tablets", 0.7 g of the dried cell powder of Lactobacillus salivarius TI 2711 strain prepared in Example 1 (cell number: 5× 10 9 CFU / g), 47 g of erythritol, 47 g of sorbitol, 2.5 g of 1-menthol, 1.5 g of flavoring agent (lime) and 1.3 g of fatty acid sugar esters as a food additive in Japan as a lubricant, Add them together and mix them uniformly, and compress the mixture into a mold with a tablet manufacturing equipment to obtain 95 grams of ordinary tablets (300 mg per tablet).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com