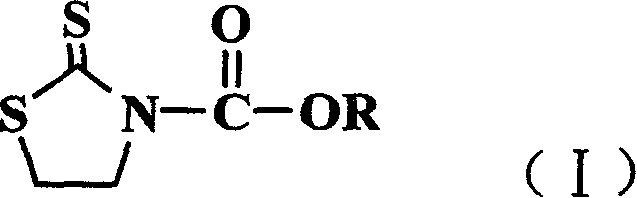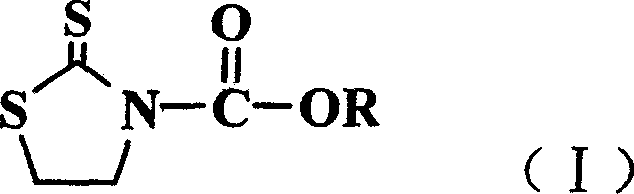Method for preparing N-alkoxy carbonyl, thiazolethioketone derivative,s and usage
A technology of oxycarbonyl and thiazolethione, applied in the field of N-oxycarbonyl-2-thiazolethione derivatives, can solve the biological activity research of rare N-oxycarbonyl-2-thiazolethione derivatives And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Synthesis of N-pentyloxycarbonyl-2-thiazolethione
[0025] Add 19.8g (0.067mol) of bis(trichloromethyl)carbonate and 50ml of dichloromethane into a 250ml three-necked flask, and slowly add n-amyl alcohol (0.2mol) under stirring. Under cooling in an ice bath, a solution of triethylamine (0.2 mol) and 10 ml of dichloromethane was slowly added dropwise, maintaining the temperature between 0 and 5°C. After the dropwise addition was completed, the temperature was raised slowly and stirred at 20° C. for 2 hours. Wash with water three times (3×50ml), dry over anhydrous sodium sulfate, filter, concentrate, and distill out n-pentyl chloroformate under reduced pressure. Yield 85.6%.
[0026] Tetrahydrothiazole-2-thione (0.60 g, 5 mmol) and triethylamine (0.72 g, 7 mmol) were dissolved in 10 ml of dichloromethane and stirred. Under cooling in an ice bath, the above n-amyl chloroformate (6 mmol) was added dropwise. After the dropwise addition, react at 0-10°C for 4 ho...
Embodiment 2
[0030] Embodiment 2 Synthesis of N-isoamyloxycarbonyl-2-thiazolethione
[0031] Add 19.8 g (0.067 mol) of bis(trichloromethyl) carbonate and 50 ml of chloroform into a 250 ml three-neck flask, and slowly add isoamyl alcohol (0.2 mol) while stirring. Under cooling in an ice bath, a solution of pyridine (0.2 mol) and 10 ml of chloroform was slowly added dropwise, maintaining the temperature between 0 and 5°C. After the dropwise addition was completed, the temperature was raised slowly and stirred at 25° C. for 2 hours. Wash with water three times (3×50ml), dry over anhydrous sodium sulfate, filter, concentrate, and evaporate isoamyl chloroformate under reduced pressure. Yield 82.7%.
[0032] Tetrahydrothiazole-2-thione (0.60 g, 5 mmol) and triethylamine (0.82 g, 8 mmol) were dissolved in 15 ml of dichloromethane and stirred. Under cooling in an ice bath, the above-mentioned isoamyl chloroformate (6 mmol) was added dropwise. After the dropwise addition, react at 0-10°C for 10...
Embodiment 3
[0036] Example 3 Synthesis of N-n-butoxycarbonyl-2-thiazolethione
[0037] N-pentanol is replaced by n-butanol, and others are the same as in Example 1 to obtain n-butyl chloroformate. Yield 88.3%.
[0038] Tetrahydrothiazole-2-thione (0.60 g, 5 mmol) and pyridine (0.56 g, 7 mmol) were dissolved in 10 ml of chloroform and stirred. Under cooling in an ice bath, the above n-butyl chloroformate (6 mmol) was added dropwise. After the dropwise addition, react at 0-10°C for 3 hours. Washed three times with water, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was separated and purified by column chromatography [V (petroleum ether): V (ethyl acetate) = 3: 1] to obtain 0.95 g of N-butoxycarbonyl-2-thiazolethione as a yellow transparent oil. The yield was 87.0%.
[0039] of the compound 1 H NMR and IR are as follows:
[0040] 1 H NMR (CDCl 3 )δ: 0.95(t, 3H, J=4.8Hz, -CH 2 CH 2 CH 2 CH 3 ), 1.41~1.47 (m, 2H, -CH 2 CH 2 CH 2 CH 3 ), 1.69~1.73 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap