Defected slow virus vector derived from HIV-1 virus
A lentiviral vector, HIV-1 technology, applied in the direction of biochemical equipment and methods, applications, botany equipment and methods, etc., can solve the problems of narrow host range, low virus titer, poor biological safety, etc., so that it is not easy to induce , good biological safety, stable expression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
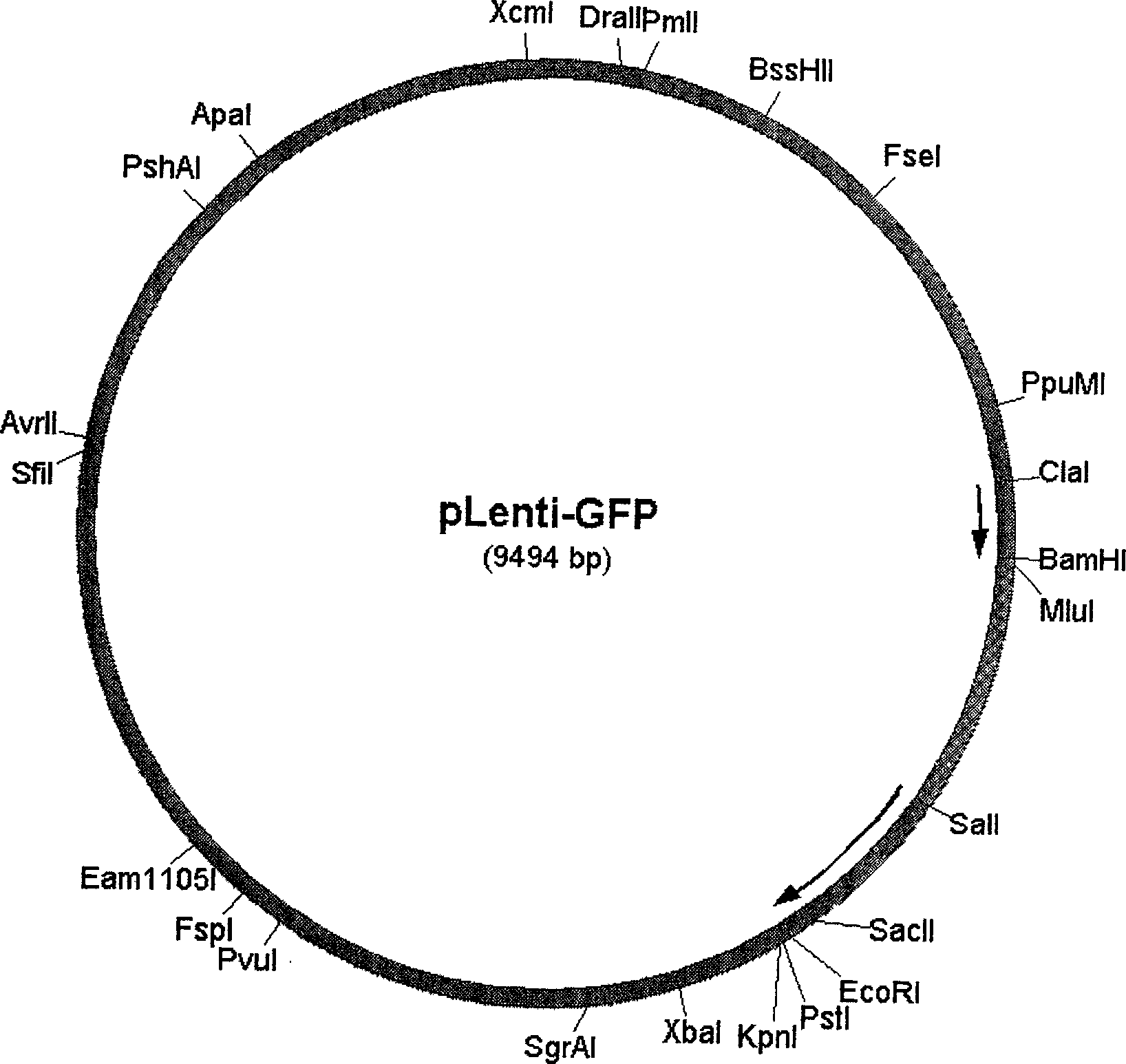
[0023] Example 1. Construction of pLenti-GFP.
[0024] (1), using the lentiviral vector pLXB isolated from the sequence of the cis-acting element (such as packaging signal, long terminal repeat sequence) and trans-acting protein in the HIV-1 genome as the original vector, it was used Xba I and Pst I digested, recovered the large fragment, digested the pHit-Sin vector with the same double restriction enzyme, obtained the 3′-LTR fragment with the U3 region removed, and connected it with T4 DNA ligase to obtain pLXB-Sin.
[0025] (2), digest pLXB-Sin with Pst I and BamH I, and reclaim the large fragment; cut the WPRE fragment from pSK-WPRE with Pst I and Sal I enzymes, and use Sal I and BamH I to cut EGFP from pEGFP-C1 Cut up and down, and then connect the above three fragments with T4 DNA ligase to obtain pLenti-GFP (structure as figure 1 ), the insertion was confirmed by restriction enzyme digestion.
Embodiment 2
[0026] Example 2. Production of pLenti-GFP lentiviral supernatant.
[0027] ①. Inoculate 10 5 293T cells were subcultured at a rate of 1:3 when the cells grew to 70-80% confluence, and DMEM (purchased from GIBCO-BRL Company) containing 10% calf serum (purchased from Hangzhou Sijiqing Company) was used as a culture medium. Cultivate for 24 hours in a 5% CO2 incubator.
[0028] ②. When the cells reach 70% confluence, transfect the pLenti-GFP plasmid lentiviral vector and the packaging plasmid with the Tat element removed by calcium phosphate co-precipitation method.
[0029] ③. Change the cell culture medium after the cells have grown for 24 hours, and culture in a 5% CO2 incubator at 37°C. Harvest the virus supernatant after 24 hours and change the cell culture medium.
[0030] ④. Harvest the virus supernatant 72 hours after transformation, and treat the cell culture dish with bleach to destroy the virus producing cells.
[0031] 5. Ultracentrifuge the virus supernatant at 5...
Embodiment 3
[0035] Identification of embodiment 3 transfection efficiency
[0036]Inoculate 10 in a 100 mm culture dish (Corning) 6 Hela cells were cultured in 10 ml of DMEM medium (purchased from GIBCO-BRL Company) containing 10% fetal bovine serum (purchased from Hangzhou Sijiqing), and subcultured at 1:3 when the cells were 80% confluent.
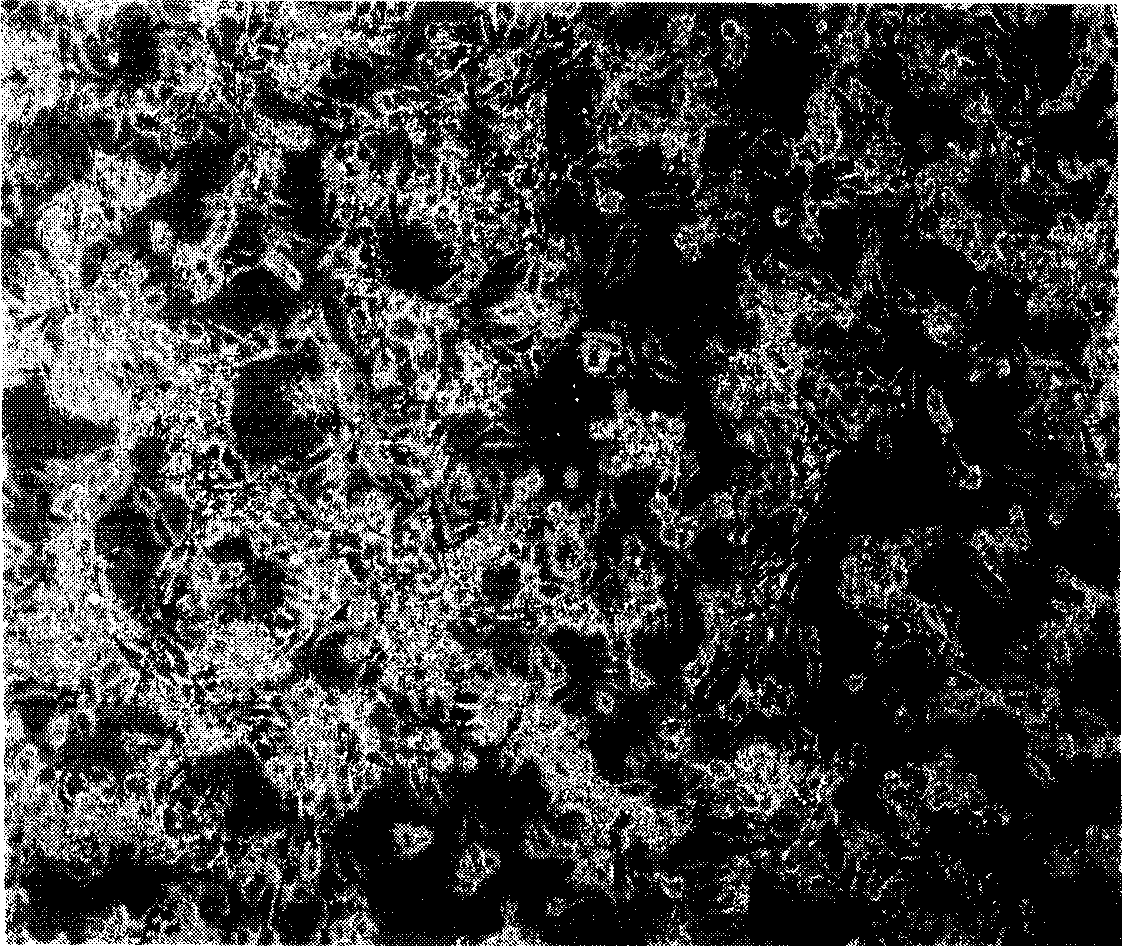
[0037] After 24 hours, suck out the medium, add 5ml of supernatant, culture in a 37°C incubator for 3h, and then supplement with 5ml of fresh DMEM medium; after 24h, perform pLenti-GFP lentivirus infection once in the same way, culture in a 37°C incubator for 24h, and then subculture , photographed with a fluorescence microscope. image 3 Fluorescent photo with figure 2 A comparison shows that almost 100% of cells are green cells after lentiviral vector infection, and the infection efficiency is nearly 100%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap