Naphthylene derivatives as cytochrome P450 inhibitors
A methyl and compound technology, applied in the field of novel heteroaryl-naphthyl-alkylamines and salts thereof, can solve the problems of limitation, lack of specificity, insufficient consideration of activity/toxicity ratio, etc.
Inactive Publication Date: 2010-10-27
OSI PHARMA INC
View PDF42 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Liarozole and other Cyp26 inhibitors inhibit other cytochrome P450-mediated responses and are therefore limited by their lack of specificity for other cytochrome P450 enzymes
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
Embodiment 3-1a
Embodiment 3-1b
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Login to View More
Abstract
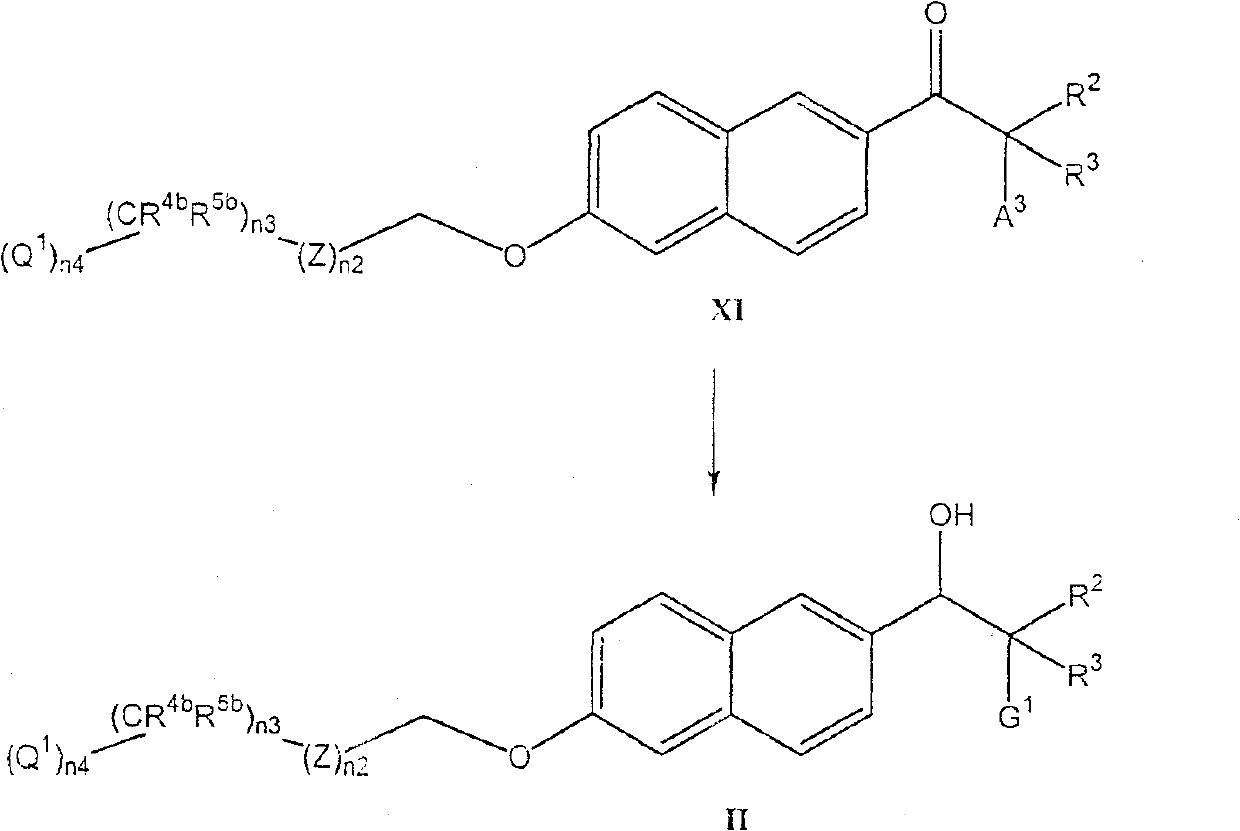
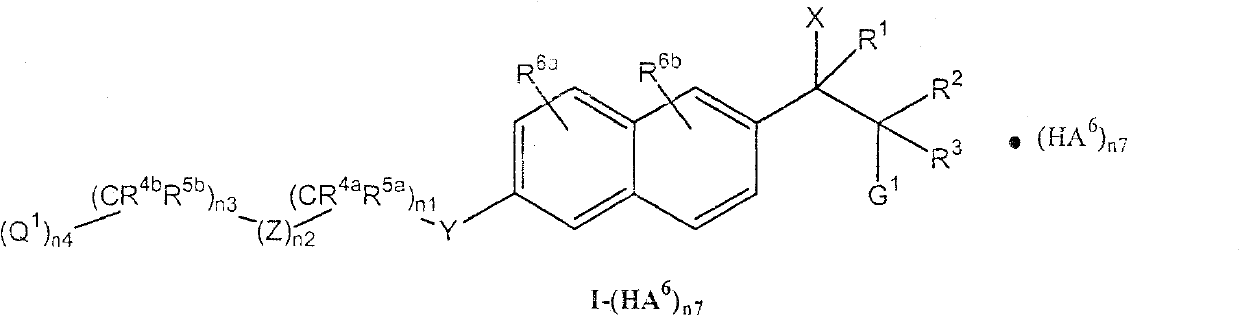
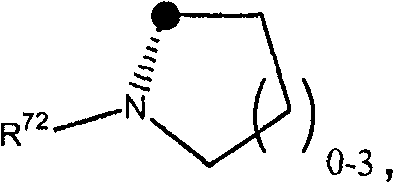
Compounds of the formula (I); and pharmaceutically acceptable salts thereof, wherein n1, n2, n3, n4, G <1>,Q<1>, Z, R<1>, R<2>, R<3>, R<4a>, R<4b>, R<5a>, and R<5b> are defined herein, inhibit the cytochrome P450RAI enzyme and are useful for the treatment and / or prevention of various diseases and conditions which respond to treatment by retinoids and by naturally occurring retinoic acid.
Description
Background of the invention The present invention relates to novel heteroaryl-naphthyl-alkylamines and salts thereof, processes for their preparation and compositions comprising them. The novel compounds of the present invention are useful for inhibiting the cytochrome P450 RAI enzyme (Cyp26) in animals, including humans, for the treatment and / or prevention of multiple diseases that respond to treatment with retinoids and naturally occurring retinoic acids. diseases and conditions. It is known in the art that retinoic acid, retinoids, and pharmaceutical compositions including retinoic acid or retinoids as active ingredients play an important role in the regulation and differentiation of epithelial cells. Such regulatory and differentiation effects, including the ability to promote cell differentiation, apoptosis, and inhibit cell proliferation, make retinoic acid and retinoid compounds useful agents in the treatment of tumors and, for example, in the treatment of skin-related...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61P35/00C07D249/04C07C211/19C07D233/54A61K31/4192A61K31/4164
CPCC07D233/54C07D249/04A61P17/00A61P17/06A61P35/00A61P43/00C07D233/64A61K31/4164A61K31/4192
Inventor 瓦内萨·史密斯安托尼·尼格罗马克·米维希尔卡拉·切萨里奥帕特里夏·安妮·贝克阿林多·卢卡斯·卡斯特拉诺
Owner OSI PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com