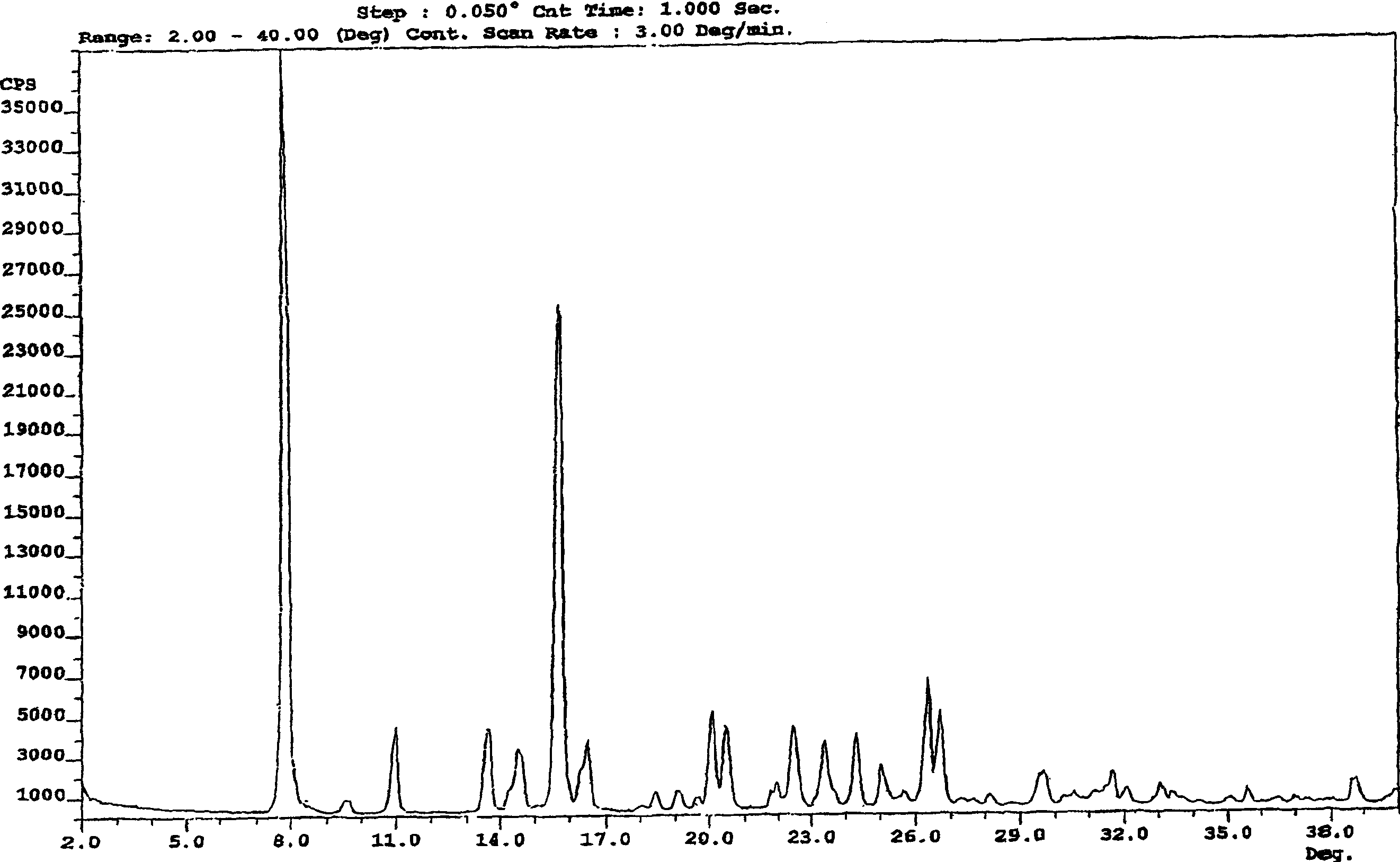
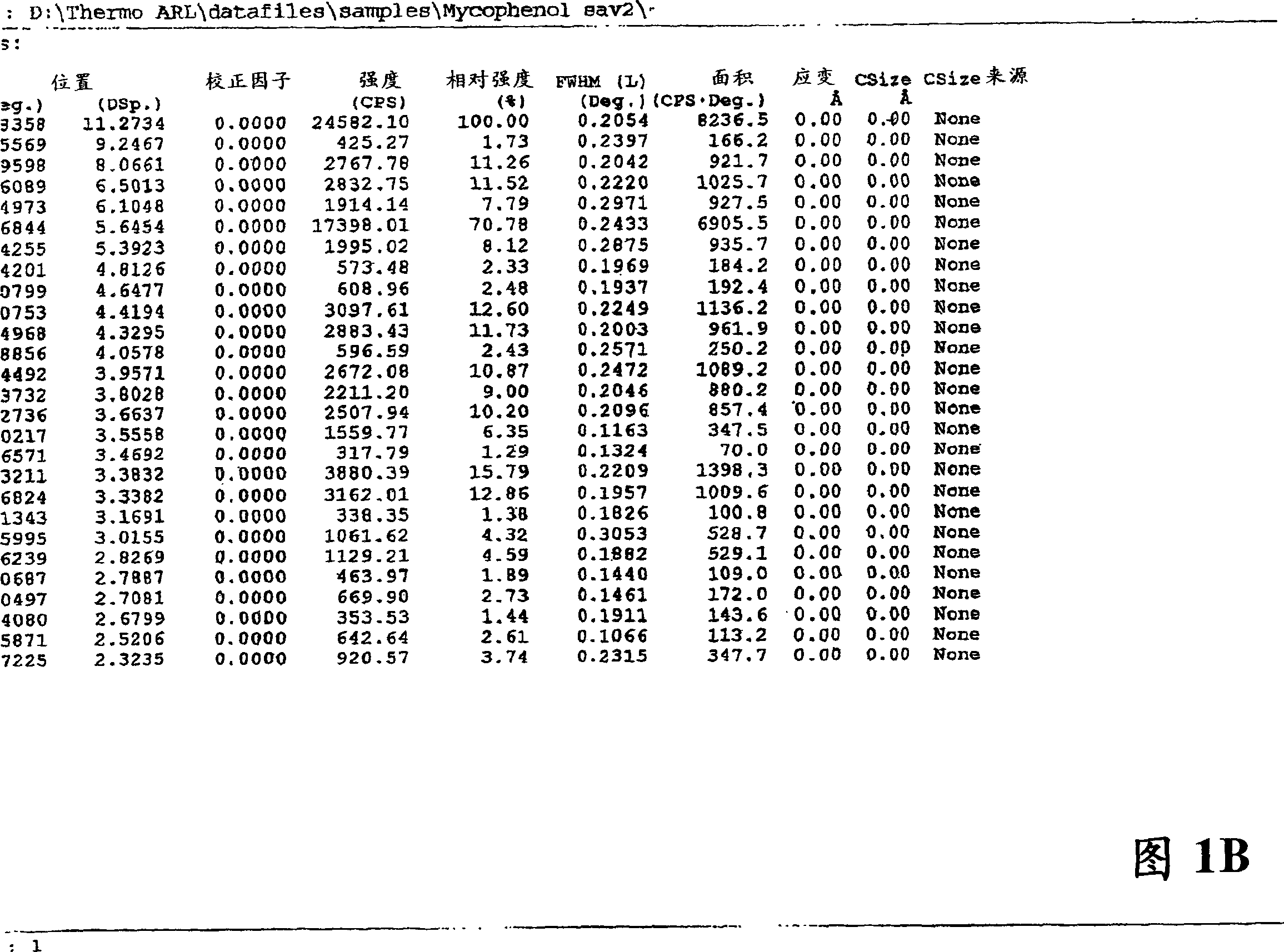
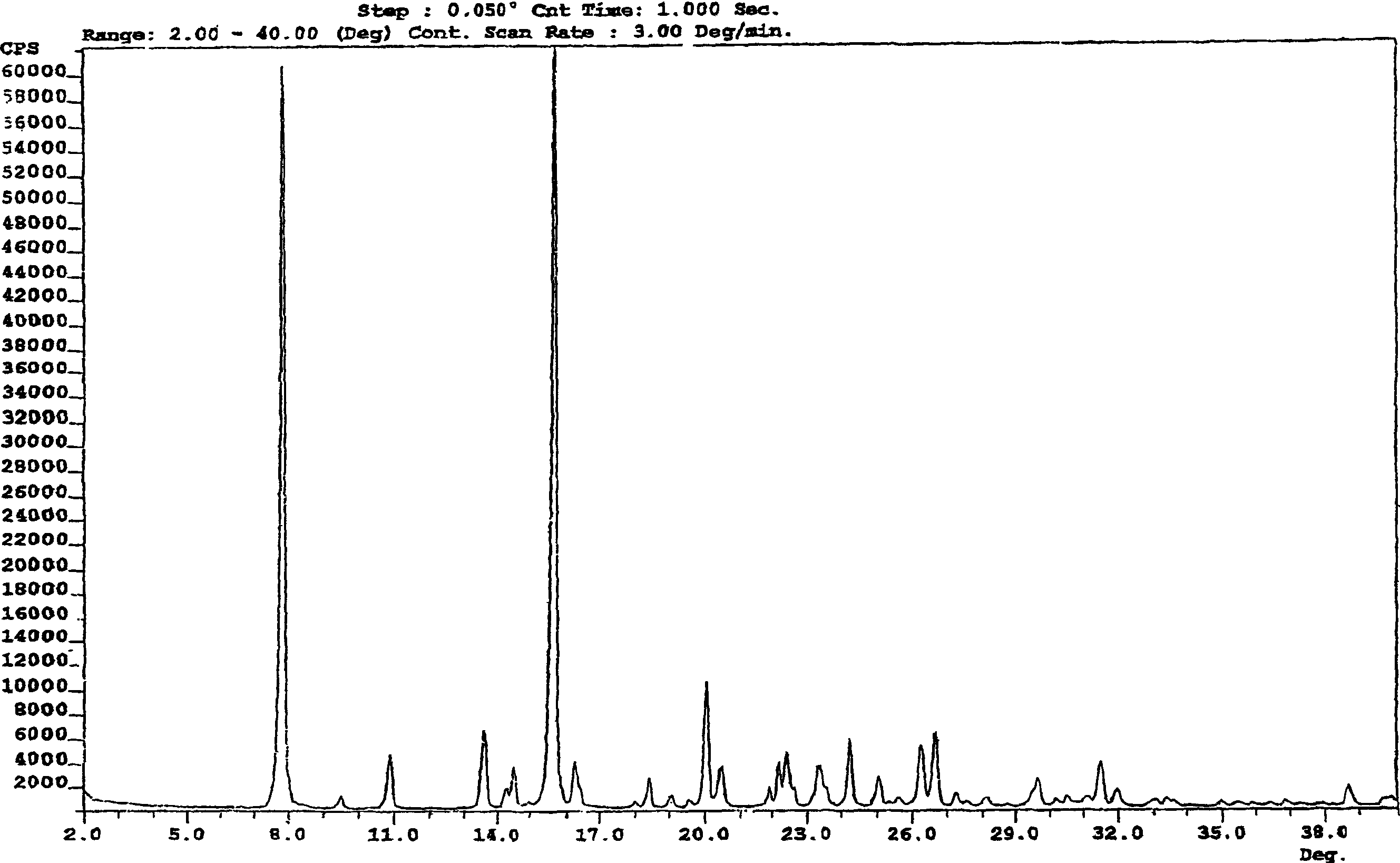
Process for preparation of mycophenolic acid and ester derivatives thereof
A mycophenolic acid, acidic technology, applied in the field of separation of mycophenolic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1: the purification of mycophenolic acid
[0095] 140 kg of concentrated mycophenolic acid suspension (produced from 620 kg of fermentation broth) was adjusted to a pH of 8.3-8.5 using 800 ml of concentrated ammonia solution. The basic solution was purified using 80 L of ethyl acetate. The ethyl acetate was mixed with the basic solution, stirred for 30 minutes and the phases were separated.
[0096] To the obtained aqueous phase (147 kg) was added 80 L of ethyl acetate. The pH was adjusted to 5.8 with sulfuric acid, stirred for 30 minutes and the phases were separated.
[0097] To the obtained aqueous phase (150 kg) was added 40 L of ethyl acetate. The pH was adjusted to 5.9, stirred for 30 minutes and the phases were separated.
[0098] The ethyl acetate phases of the two acidic extractions are combined and concentrated under reduced pressure at a maximum of 70° C. to a concentration of about 200 g / l. The concentrated ethyl acetate solution was heated t...
Embodiment 2
[0100] Embodiment 2: the purification of mycophenolic acid
[0101] 71.6 L of ethyl acetate was added to 119.4 kg of concentrated mycophenolic acid suspension (produced from 420 kg of fermentation broth). The pH was adjusted to 9.1 with concentrated ammonia, stirred for 30 minutes, and the phases were separated.
[0102] To the aqueous phase (126.8 kg) was added 63.4 L of ethyl acetate. The pH was adjusted to 9.1-9.2 using concentrated ammonia, stirred for 30 minutes, and the phases were separated.
[0103] To the obtained aqueous phase (129.5 kg) was added 71.6 L of ethyl acetate. The pH was adjusted to 5.6-5.7 using sulfuric acid, stirred for 30 minutes and the phases were separated.
[0104] To the obtained aqueous phase (130.6 kg) was added 39.2 L of ethyl acetate. The pH was adjusted to 5.9 using concentrated ammonia, stirred for 30 minutes and the phases were separated.
[0105] The ethyl acetate phases of the two acidic extractions are combined and concentrated und...
Embodiment 3
[0108] Embodiment 3: the preparation of concentrated mycophenolic acid
[0109] The pH of the fermentation broth (15 kg) was adjusted to 8.0-11.0. The alkaline fermentation broth is filtered, and the filtered mycelium is washed with water. The filtrate was 37.6 kg. 91.1% of fermentation active ingredients were obtained in the filtrate. The pH of the filtrate was adjusted to 2.0-2.2 using sulfuric acid. Add filter aid (perlite) to the acidic filtrate and filter out the precipitate. The filtered precipitate was suspended in 5 L of water, and the pH of the suspension was adjusted to pH 8.0-11.0 using sodium hydroxide solution. The basic suspension was filtered and washed to obtain 8 L of basic filtrate.
[0110] This alkaline suspension is used for purifying mycophenolic acid, as in Example 1 or 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com