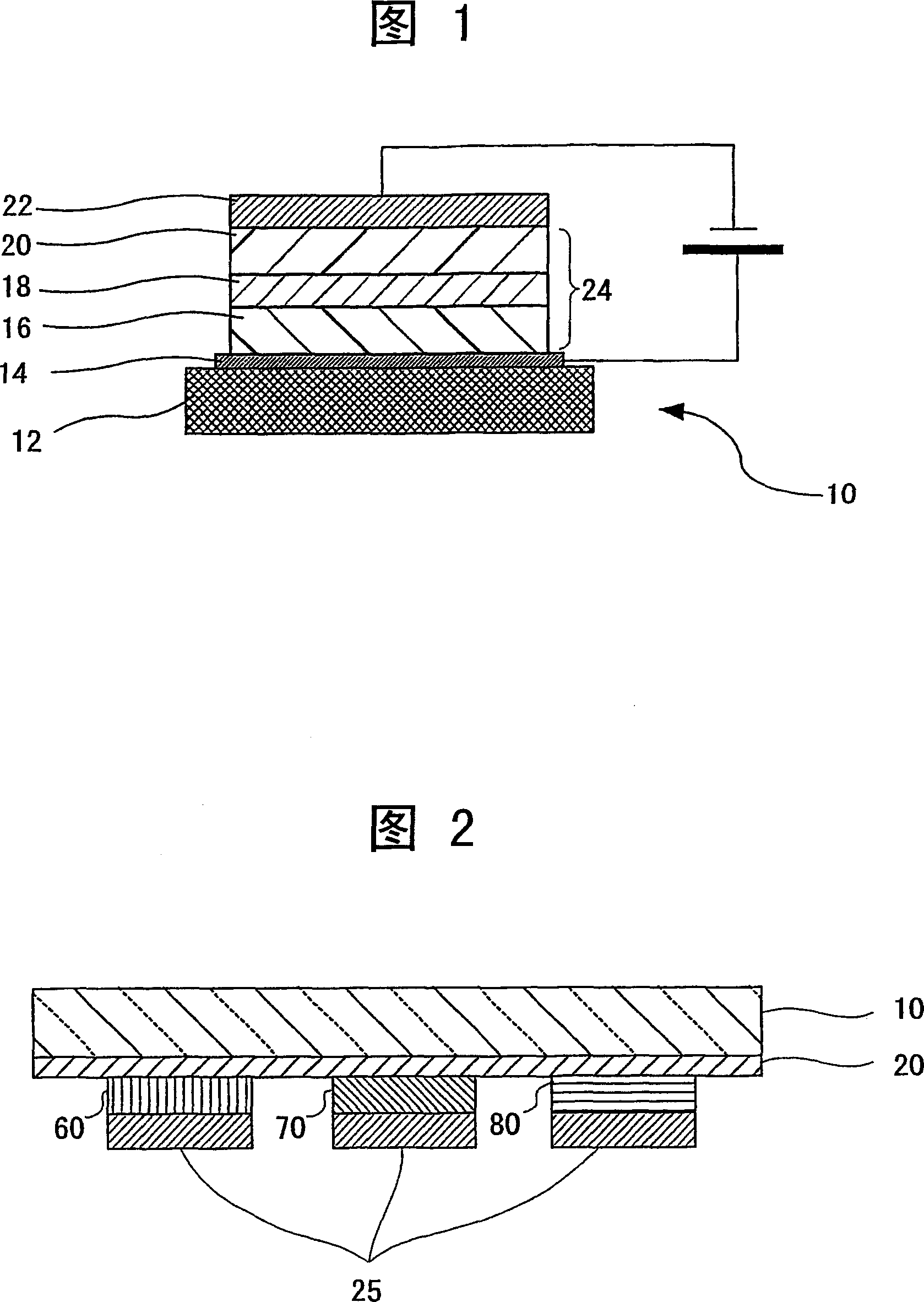
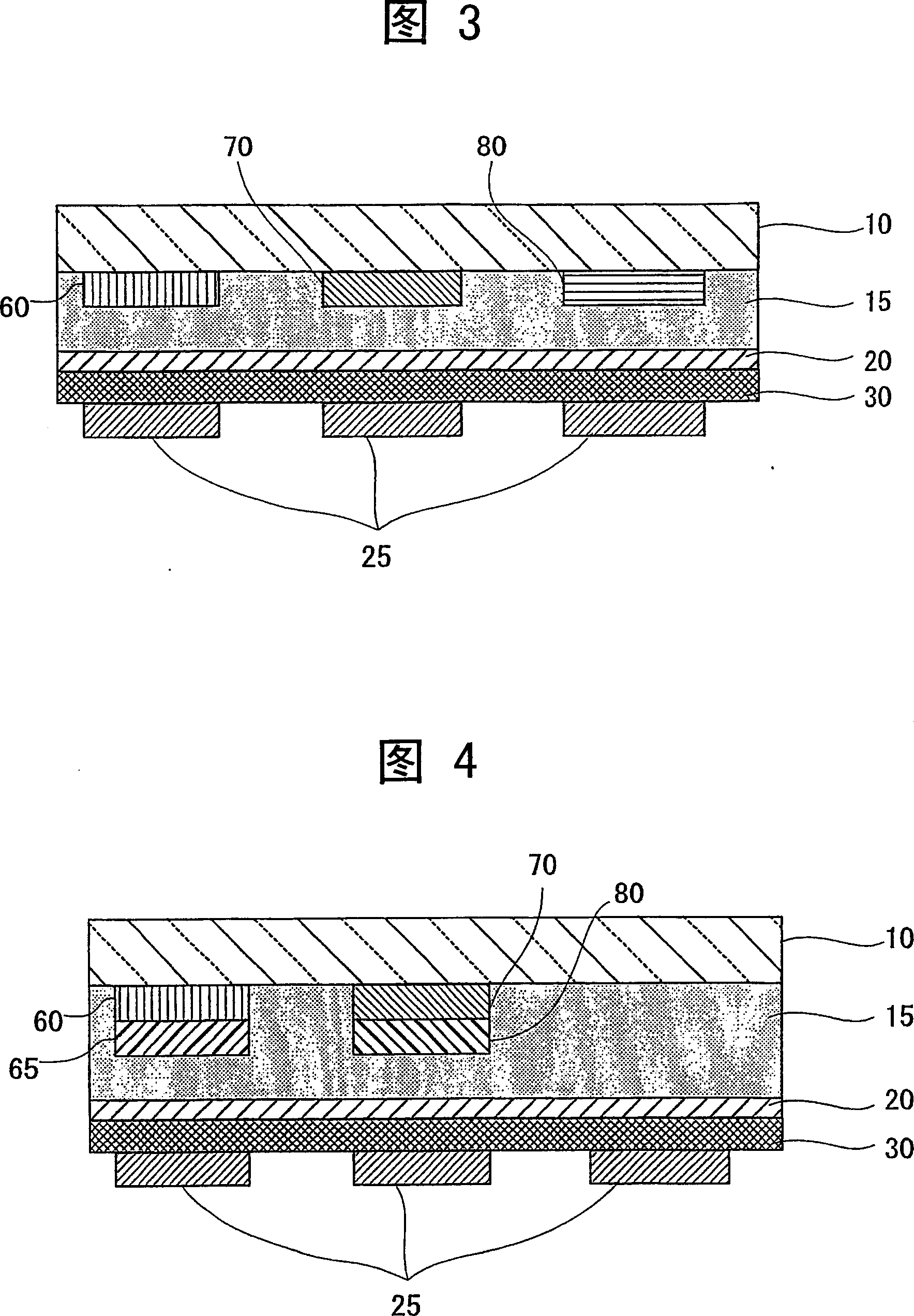
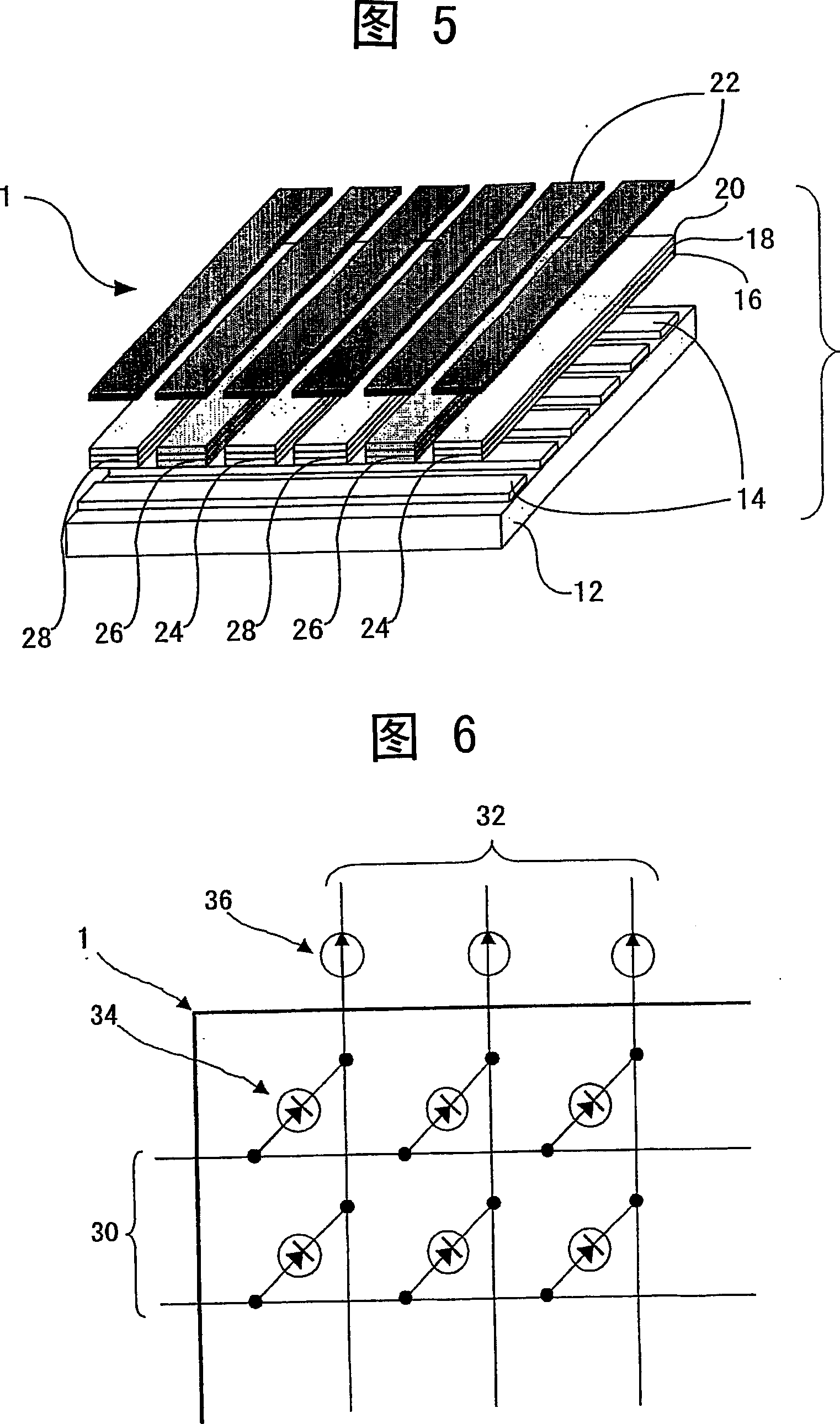
Organometallic complex, luminous solid, organic EL element and organic EL display
An organometallic and complex technology, which is applied in the field of luminescent solids and organometallic complexes, can solve the problems of less materials, short driving life, and narrow range of material selection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0230] Synthesis Example 1: Synthesis of Pt(3,5-bis(2-pyridyl)toluene)(biphenyl oxide) (hereinafter referred to as "Pt(dpt)(obp)")
[0231] Pt(3,5-bis(2-pyridyl)toluene)(biphenyl oxide) (hereinafter referred to as "Pt(dpt)(obp)") was synthesized as follows. That is, specifically, 3,5-dibromotoluene (5.0 g; 20 mmol), 2-tri-n-butylstannylpyridine (26.9 g; 73 mmol), bis(triphenylphosphine) Palladium dichloride (1.55 g; 2.2 mmol) and lithium chloride (11.7 g; 276 mmol), refluxed for 2 days. After cooling, 50 ml of a saturated aqueous solution of KF was added. The precipitated solid was taken out by filtration, washed with a small amount of cooled toluene (20ml x 3 times), and dried in vacuo. The resulting solid was added to dichloromethane and NaHCO 3 In the mixed solution, wash thoroughly. The organic layer was separated with MgSO 4 After drying, the solvent was removed using an evaporator. Recrystallization was performed with dichloromethane to obtain 2.2 g of 3,5-bis(2-py...
Synthetic example 2
[0239] Synthesis Example 2: Synthesis of Pt(3,5-bis(2-pyridyl)toluene)(OH) (hereinafter referred to as "Pt(dpt)(OH)")
[0240] After obtaining Pt(dpt)Cl in the same manner as in Synthesis Example 1, 100 mg (0.21 mmol) of the obtained Pt(dpt)Cl was added to 30 ml of acetone and stirred. 56 mg (1 mmol) of KOH powder was added thereto, and stirred at room temperature for 10 minutes. After adding a few drops of pure water, a yellow solid began to precipitate. Stir while heating for 3 hours, let cool, filter out the precipitated solid, wash thoroughly with pure water, methanol, and diethyl ether in sequence, and then vacuum-dry to obtain a yellow solid of Pt(dpt)(OH). The yield is 68%. The IR spectrum of Pt(dpt)(OH) is shown in FIG. 10 .
[0241]
Synthetic example 3
[0242] Synthesis Example 3: Synthesis of Pt(3,5-bis(2-pyridyl)toluene)(1,2,4-triazole salt) (hereinafter referred to as "Pt(dpt)(taz)")
[0243] After obtaining Pt(dpt)Cl in the same manner as in Synthesis Example 1, 100 mg (0.21 mmol) of the obtained Pt(dpt)Cl was added to 30 ml of acetone and stirred. 29 mg (0.32 mmol) of 1,2,4-triazole sodium salt was added thereto, and stirred at room temperature for 10 minutes. After adding a few drops of pure water, a yellow solid began to precipitate. Stir while heating for 3 hours, let cool, filter out the precipitated solid, wash thoroughly with pure water, methanol, and diethyl ether in sequence, and then vacuum-dry to obtain a yellow solid of Pt(dpt)(taz). The yield was 82%. The IR spectrum of Pt(dpt)(taz) is shown in FIG. 11 .
[0244]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap