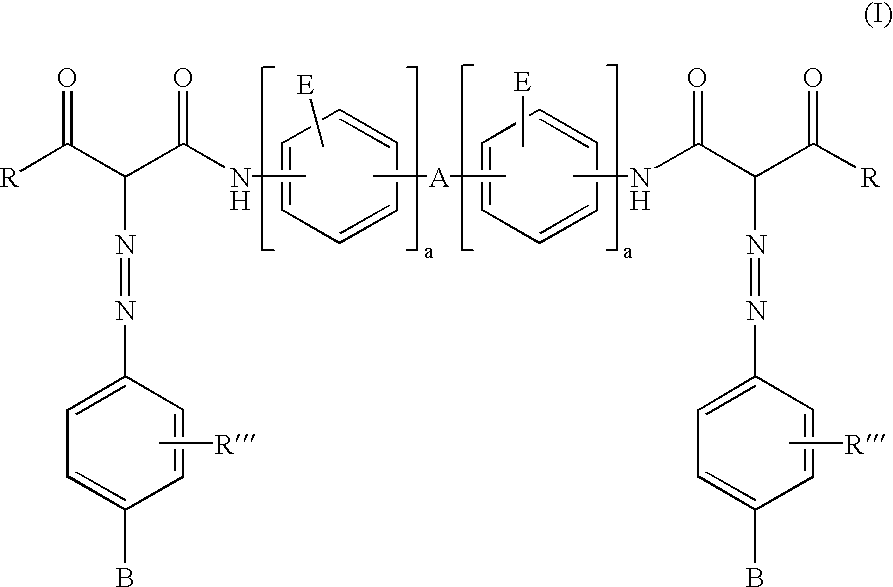
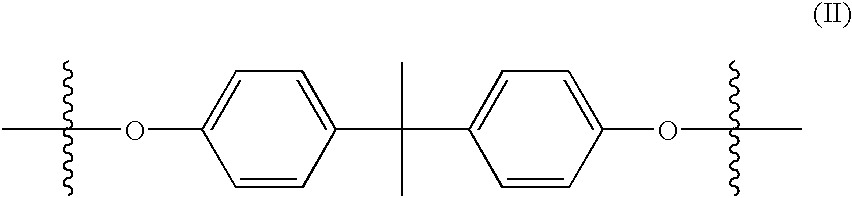
Polymeric bis-acetoacetanilide azo colorants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] 9
[0029] Ethanol (200 proof, 515 g) and p-aminophenol (300 g, 2.75 mol) were charged into a 2 L 3-neck round bottom flask equipped with a thermometer and a condenser. Benzaldehyde (292 g, 2.75 mol) was added from an additional funnel while maintaining a slow and steady flow and stirring. Another portion of ethanol (150 g) was added and the whole mixture was heated to reflux for 1.5 h. After cooling down to room temperature, the solid thus formed was collected by filtration and washed three times with ethanol (3.times.50 ml) and dried. 492 g (91%) of the imine product (structure above) was obtained as a pale yellow powder.
example 2
[0030] 10
[0031] The imine product (285 g, 1.44 mol) from Example 1, toluene (200 ml) and KOH (6 g) were charged into a one-gallon stainless steel reactor. After being purged 3 times to 60 psi with nitrogen, the reaction mixture was heated to 250.degree. F. and ethylene oxide (635 g, 14.4 mol) was added over a period of 2 hours. The mixture was then cooled down to room temperature and the toluene was stripped to yield 910 g (98.2%) of pale brown yellow liquid polyethylene glycol (10) imine (structure above).
example 3
[0032] 11
[0033] The imine (340 g, 0.27 mol) from Example 2 and water (150 g) were charged into a 1000-ml 3-neck flask. Concentrated hydrochloric acid (56 g) was added carefully while stirring and maintaining the temperature below 45.degree. C. After stirred for 30 min at 50.degree. C., the mixture was transferred into a 1000-ml flask and stripped by a rotary evaporator at 85-95.degree. C. for 1.5 hour. After cooling down to room temperature, 240 g of water was charged into the reaction mixture and the whole was stripped at 85-95.degree. C. for 1.5 hour. Then another 240 g of water was added and the mixture was stripped at 85-95.degree. C. till all of the water and benzaldehyde were removed. 285 g (97%) of product polyethylene glycol (10) aniline (structure above) was obtained as a light brownish yellow liquid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Haze | aaaaa | aaaaa |
| Haze | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap