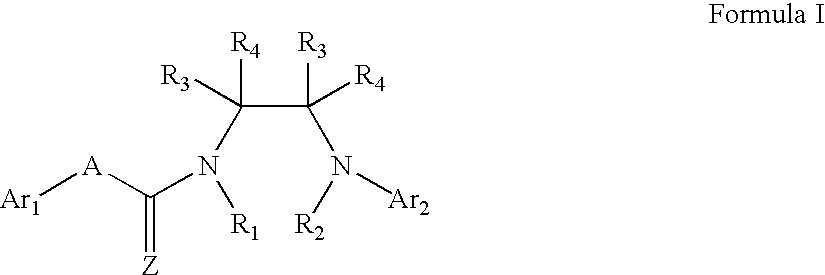
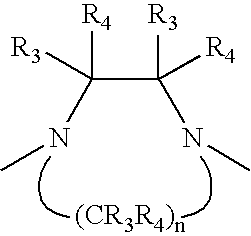
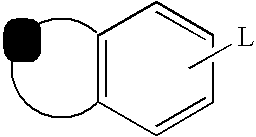
Capsaicin receptor ligands
a capsaicin receptor and ligand technology, applied in the field of compounds, can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
(R)-4-(3-Chloro-pyridin-2-yl)-2-methyl-piperazine-1-carboxylic acid (4-sec-butyl-phenyl)-amide
[0262] Part A: Synthesis of (R)-1-(3-Chloro-pyridin-2-yl)-3-methylpiperazi-ne: 26
[0263] Dissolve 2,3-dichloropyridine (8.5 g, 0.057 moles) and (R)-(-)-2-methylpiperazine (5.75 g, 0.057 moles) in N,N-dimethylacetamide (125.0 mL) under nitrogen atmosphere. Add anhydrous powdered K.sub.2CO.sub.3 (23.75 g, 0.172 moles) to this mixture and stir at 135-140.degree. C. for 48 h. New spot noticed in TLC (5% MeOH / CHCl.sub.3 / 1% NEt.sub.3) along with absence of starting materials. Cool the reaction mixture to room temperature, dilute with water (400 mL), extract with EtOAc (3.times.200 mL) and wash the combined organic extract with brine (2.times.150 mL). Dry over MgSO.sub.4, concentrate under vacuum to afford crude product (20.0 g) as orange yellow liquid. Distil the crude under high vacuum to afford pyridylpiperazine derivative as yellow viscous oil (10 g, bp 112-115.degree. C. / 0.1 torr). NMR (CDCl.s...
example 2
(R)-(-)-4-(3-Chloro-pyridin-2-yl)-2-methyl-piperazine-1-carboxylic acid (4-trifluoromethyl-phenyl)-amide
[0269] 29
[0270] Dissolve Part A material of example 1 (0.2756 g, 1.3 mmoles) in toluene (1.5 mL) under nitrogen at room temperature. Add drop wise 4-trifluoromethylphenyl isocyanate (0.2431 g, 1.3 mmoles) dissolved in toluene (50 mL) to the mixture over a period of 30 mins and stir at room temperature for 3 hours. Evaporate the solvent from reaction mixture under vacuum to afford colorless oil. Crystallize the oil from 1:1 Et.sub.2O / hexane (2.0 mL) to afford white solid.
[0271] NMR (CDCl.sub.3): .delta. 1.45-1.47 (d, 3H, J=1.7 Hz), 2.97-3.01 (t, 1H), 3.06-3.10 (m, 1H), 3.47-3.50 (m, 1H), 3.75-3.85 (m, 2H), 3.92-3.95 (m, 1H), 4.37-4.38 (m, 1H), 6.59 (bs, 1H), 6.88-6.91 (dd, 1H), 7.52-7.56 (m, 4H), 7.61-7.63 (dd, 1H), 8.19-8.21 (dd, 1H).
[0272] Mass spectrum (ESI): 399.3 (M+H).
[0273] Analysis calcd. for C.sub.18H.sub.18ClF.sub.3N.sub.4O: C, 54.21; H, 4.55; Cl, 8.89; F, 14.29; N, 14.05...
example 3
(R)-3-Chloro-pyridin-2-yl)-2-methyl-piperazine-1-carboxylic acid 4-tert-butyl-phenyl ester
[0274] Part A: Synthesis of (R)-4-(3-Chloro-pyridin-2-yl)-2-methylpiperazi-ne-1-carbonyl chloride: 30
[0275] Dissolve Part A material of Example 1 (1.06 g, 5.0 mmole)) in CH.sub.2Cl.sub.2 (50 mL) and saturated NaHCO.sub.3 (50 mL) under nitrogen at room temperature. Add drop wise 20% COCl.sub.2 in toluene (5.0 mL) at room temperature and stir overnight. Separate the organic layer, extract the aq. layer with CH.sub.2Cl.sub.2 (2.times.15 mL) and dry (MgSO.sub.4). Evaporate the organic layer under vacuo to afford yellow oil.
[0276] Part B: Title Compound: 31
[0277] Dissolve Part A material of Example 3 (136 g, 0.5 mmole)) in pyridine (2.0 mL) under nitrogen at room temperature. Add 4-tert.butylphenol to the reaction mixture at room temperature and stir overnight. Evaporate the reaction mixture under vacuo, partition between water / CH.sub.2Cl.sub.2 (20 mL) and dry (MgSO.sub.4). Evaporate the organic lay...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap