Molded electrode, method for production thereof, and secondary battery using thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
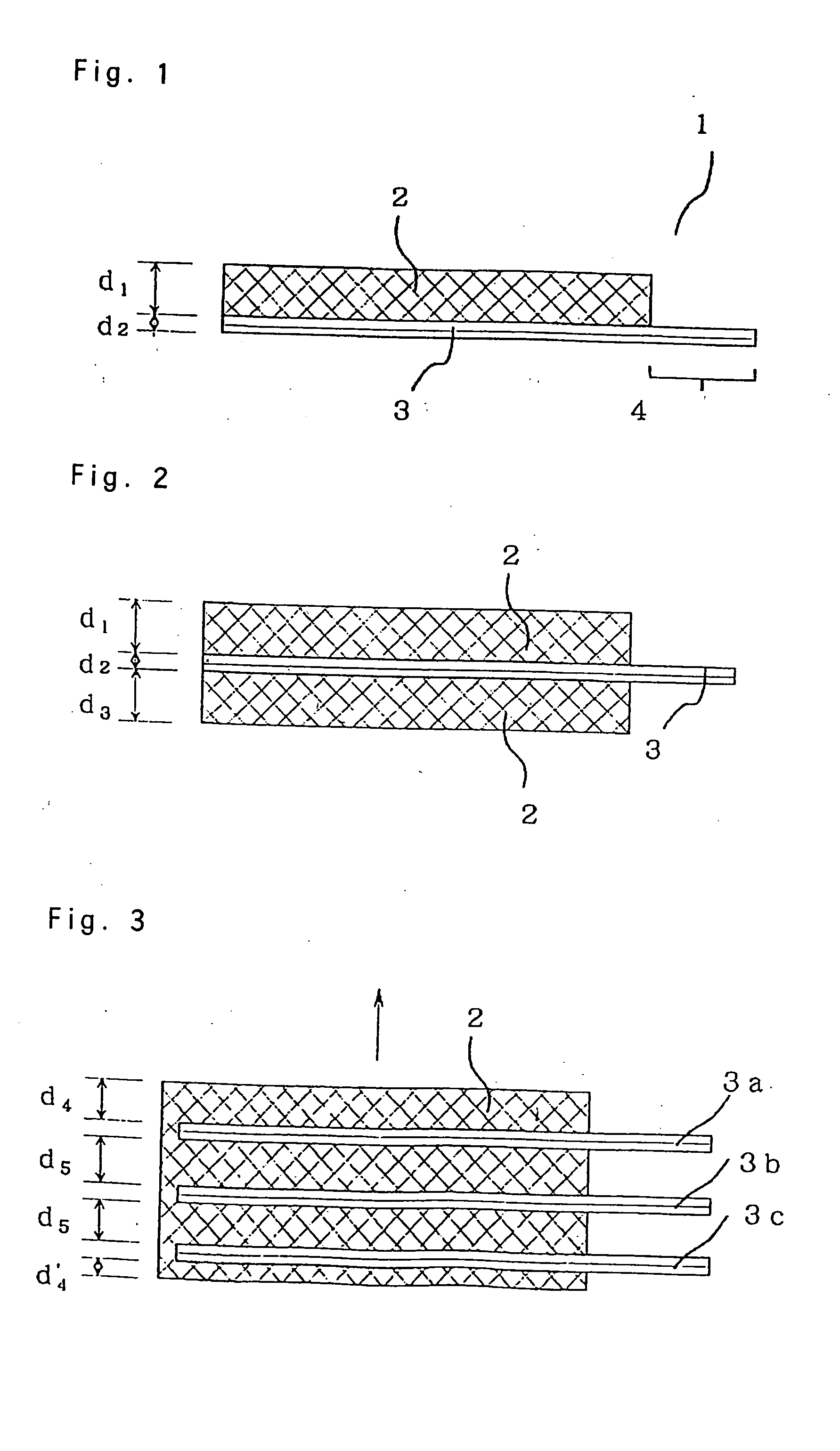
In this Example, a current collector sheet and an electrode material were hot-pressed into a one-piece molded electrode having a structure of FIG. 1; and a battery was constituted using the molded electrode as the positive electrode.
Production of Battery of Example 1
First, description is made on production of a battery in the following (1) to (4).
(1) Production of Positive Electrode Mixture
An aqueous solution of 1 M ammonium peroxodisulfate and an aqueous solution of 1 M of an aniline monomer were mixed with stirring at room temperature for 3 hours to allow a polymerization reaction to proceed slowly. The polymerization reaction product obtained was ground to 60 mesh or below using an agate mortar. Thereto was added an aqueous solution of 1 M of paratoluenesulfonic acid. The mixture was stirred at 65° C. for 12 hours to dope the polyaniline (PAn) with a paratoluenesulfonic acid anion (pTOS−), whereby a PAn / pTOS− powder having conductivity was obtained.
There were mixed the...
example 2
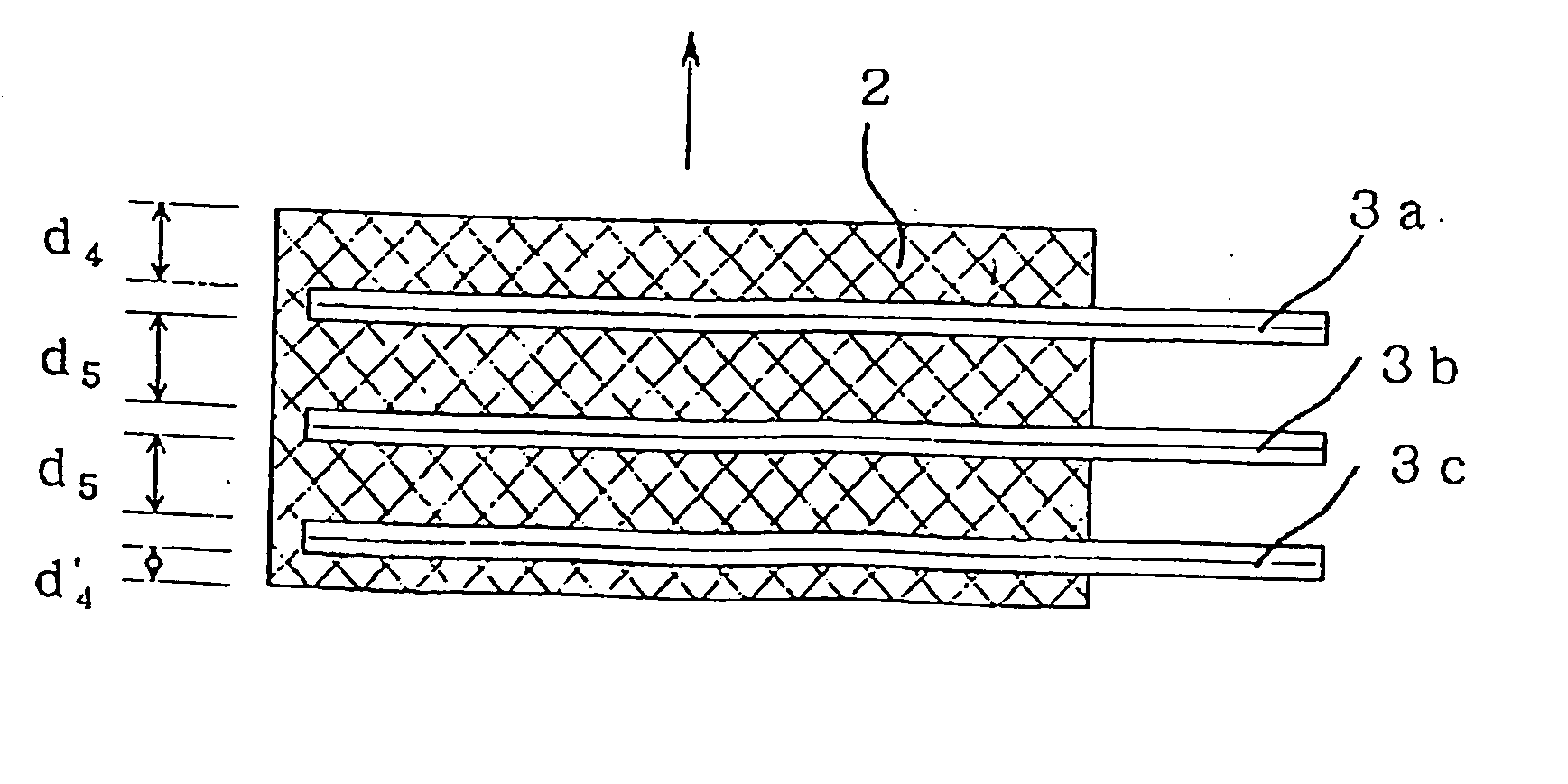
In Example 2, hot pressing for one-piece molding of current collector and electrode material was conducted 3 times to produce a molded electrode having 3 current collector sheets in the thickness direction. A battery was constituted by using the molded electrode as a positive electrode.
First, a one-piece molded positive electrode consisting of an electrode material and a current collector was formed using the same electrode material and same manner as in Example 1. With this one-piece electrode being left in the die used, the same nickel current collector as used in Example 1 was placed in the die. Then, the same positive electrode mixture as used in Example 1 was filled uniformly, and hot pressing was conducted under the same conditions as in Example 1. This operation was conducted once more to obtain a one-piece molded electrode containing three current collectors. Then, the terminal portions of the nickel current collectors were welded to form one terminal for positive electro...
example 3
The operation of Example 3 was the same as in Example 1 except that the positive electrode material was a polycyanoindole, the neutral electrolytic solution was an aqueous solution containing 1 M of zinc sulfate, and the negative electrode was metal zinc.
First, an ethanol solution of 1 M of iron sulfate and an aqueous solution of 1 M of cyanoindole were mixed with stirring at room temperature for 3 hours, to allow a polymerization reaction to proceed slowly. The polymerization product (a polycyanoindole) obtained by filtration and washing with DMF was ground to 60 mesh or below using an agate mortar. Since the polycyanoindole had been doped with sulfate ion during the polymerization reaction, no doping was applied to the polycyanoindole.
Using this polycyanoindole powder doped with sulfate ion and employing the same manner as in Example 1, a powdery electrode mixture was obtained which consisted of the polycyanoindole, a vapor phase-grown carbon and butyl butylphthalylglycolate....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
| Electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com