Methods and materials for treating and preventing inflammation of mucosal tissue
a technology of mucosal tissue and mucosal inflammation, which is applied in the field of methods and materials for treating and preventing can solve the problems of rhinosinusitis, the inflammation of mucosal tissue, and the serious medical problem that affects millions of people worldwide, and achieves the effects of reducing inflammation, preventing afs symptoms, and reducing the level of fungal organisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Collecting and Analyzing Mucus Samples
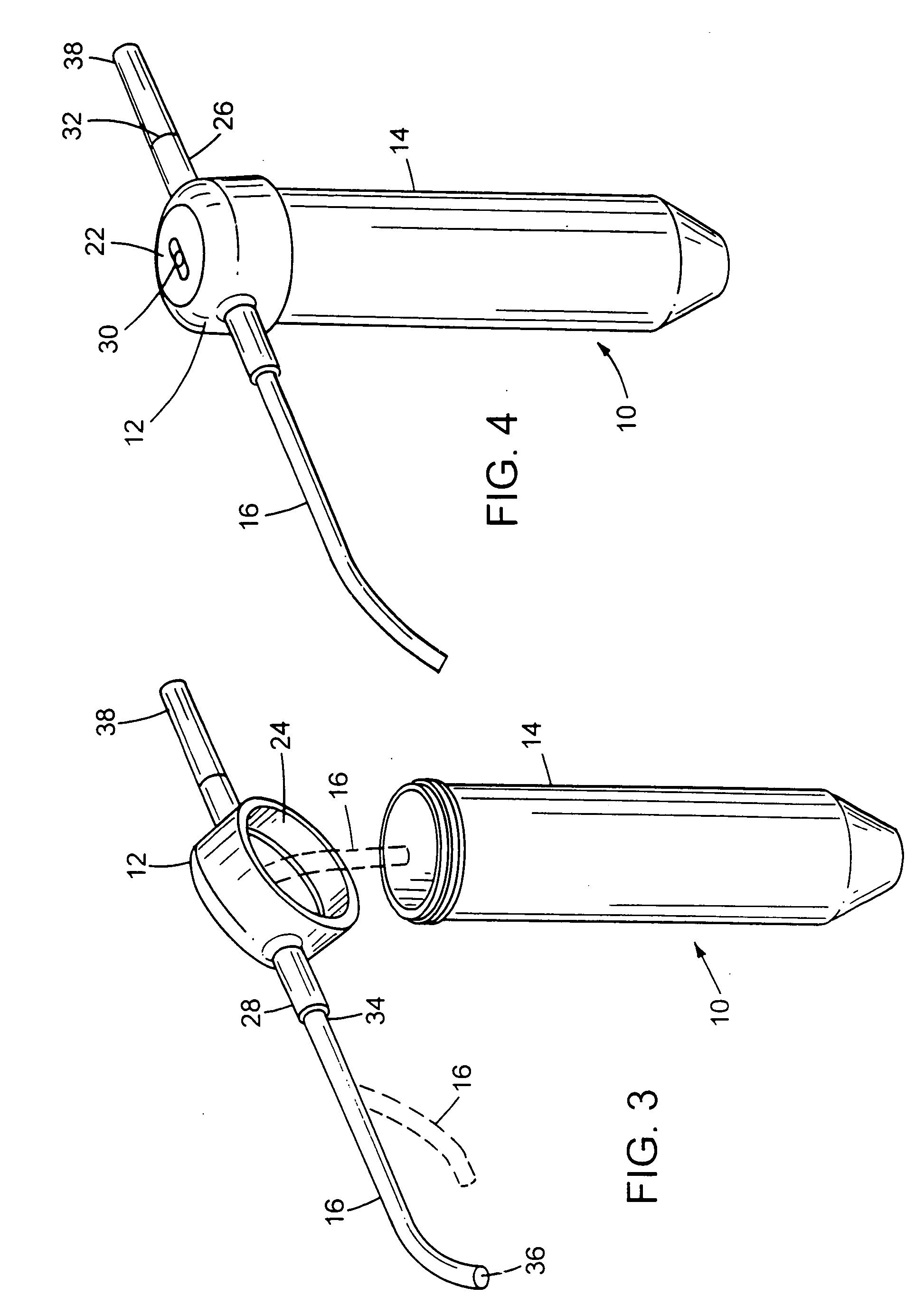
[0161] The following methods and materials were used to collect and analyze mucus from 202 patients. Prior to collecting the mucus, each patient was directed to inhale and then lower his or her chin toward their chest to minimize or prevent the flow of a collection solution out of the nasal-paranasal passageways via the normal drainage at the back of the throat. The collection solution was either a sterile saline solution or sterile water. In addition, each patient was positioned such that the flow of the collection fluid out of the nasal passageways would be minimized or prevented. Some patients received an administration of a vasoconstrictor, such as phenylephrine hydrochloride (1-2 sprays per nostril) or cocaine (topical liquid or powder; less than four mg per kg of body weight). Some patients received a spray of about three mL of a 20% solution of N-acetyl-L-cysteine. Patients receiving both were given the vasoconstrictor first and then abo...
example 2
Treating and Preventing Non-Invasive Fungus-Induced Rhinosinusitis
[0174] One hundred and thirty-two consecutive rhinosinusitis patients were entered into a study to evaluate the use of an antifungal agent to treat non-invasive fungus-induced rhinosinusitis. After diagnostic analysis, 125 of the 132 patients (95%) had the following criteria: (1) presence of observable disease within the nasal-paranasal anatomy as evidenced by a CT scan, (2) presence of allergic mucus as evidenced by histologic evaluation of a surgical specimen, and (3) presence of fungal organisms within nasal-paranasal mucus as evidenced by the ability to culture fungal organisms from a mucus sample. The 125 non-invasive fungus-induced rhinosinusitis patients were started on an antifungal treatment of about 20 mL of an amphotericin B solution per nostril, two to four times daily for at least three months. The concentration of the amphotericin B solution was about 100 mg per liter of saline or water. A 20 mL bulb wa...
example 3
Treating and Preventing Non-Invasive Fungus-Induced Rhinosinusitis in Patients without Previous Nasal Surgery
[0206] The following three non-invasive fungus-induced rhinosinusitis patients did not have a previous nasal surgery.
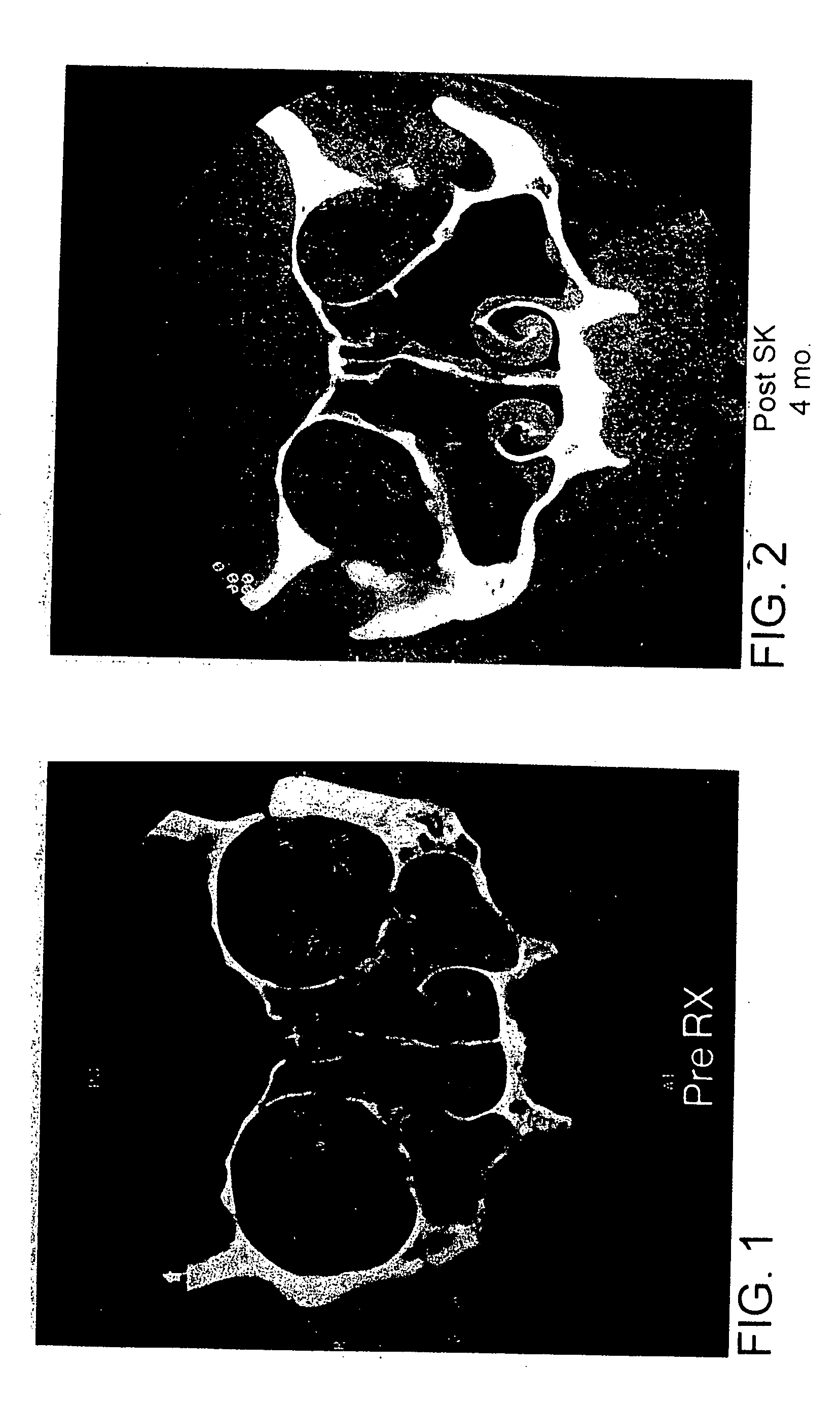
[0207] A 61 year old male was diagnosed with non-invasive fungus-induced rhinosinusitis and instructed to perform amphotericin B irrigations twice a day. Before starting the treatment, endoscopic evaluation revealed polyps filling her nasal cavity (endoscopic score 3) and the patient gave herself a symptom score of −1. After using the amphotericin B irrigations for fourteen months, endoscopic evaluation revealed no evidence of disease (endoscopic score 0) and the patient gave herself a symptom score of +2.
[0208] A 64 year old female was diagnosed with non-invasive fungus-induced rhinosinusitis and instructed to perform amphotericin B irrigations twice a day, which was later increased to four times a day. Before starting the treatment, endoscopic evaluation r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com