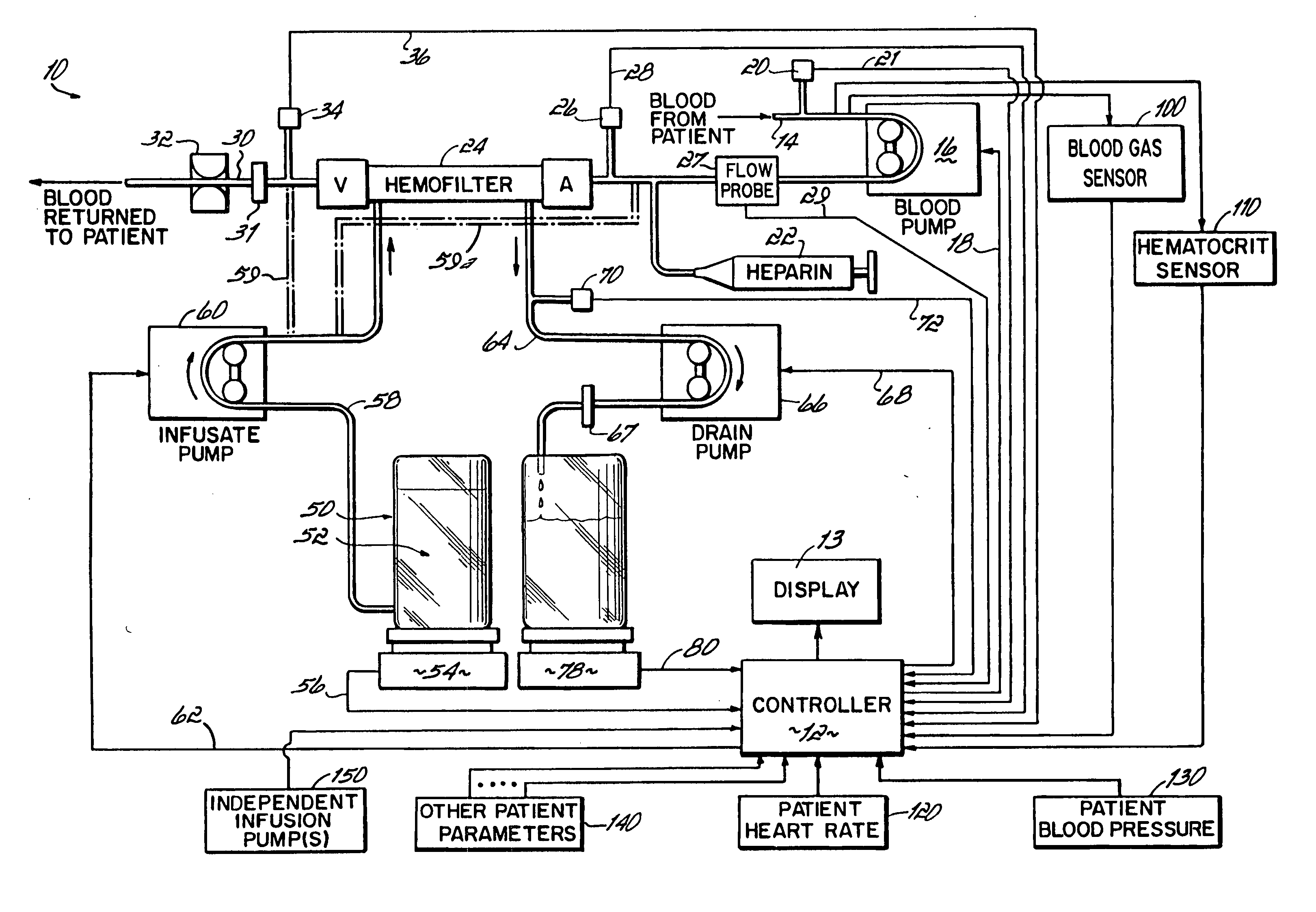
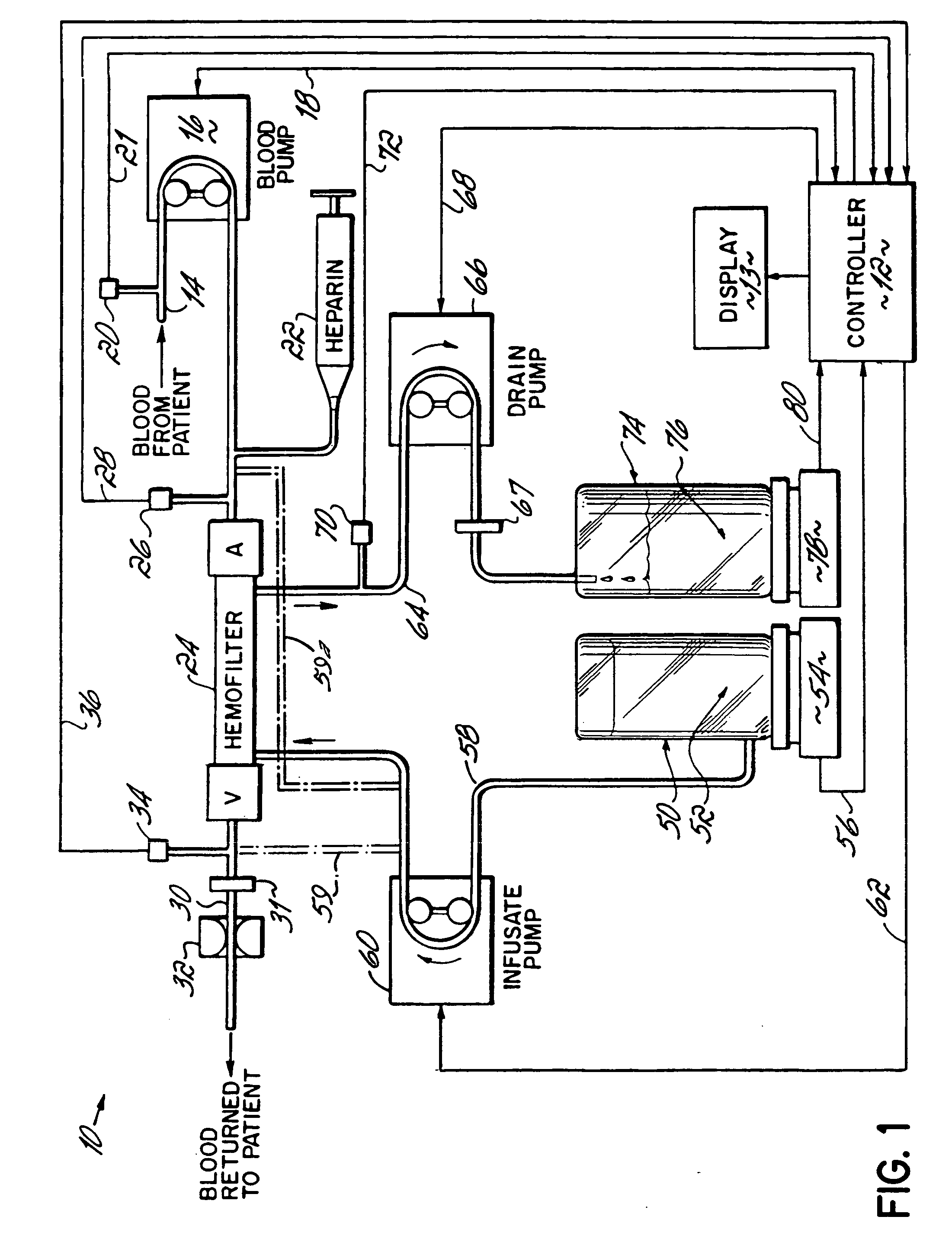
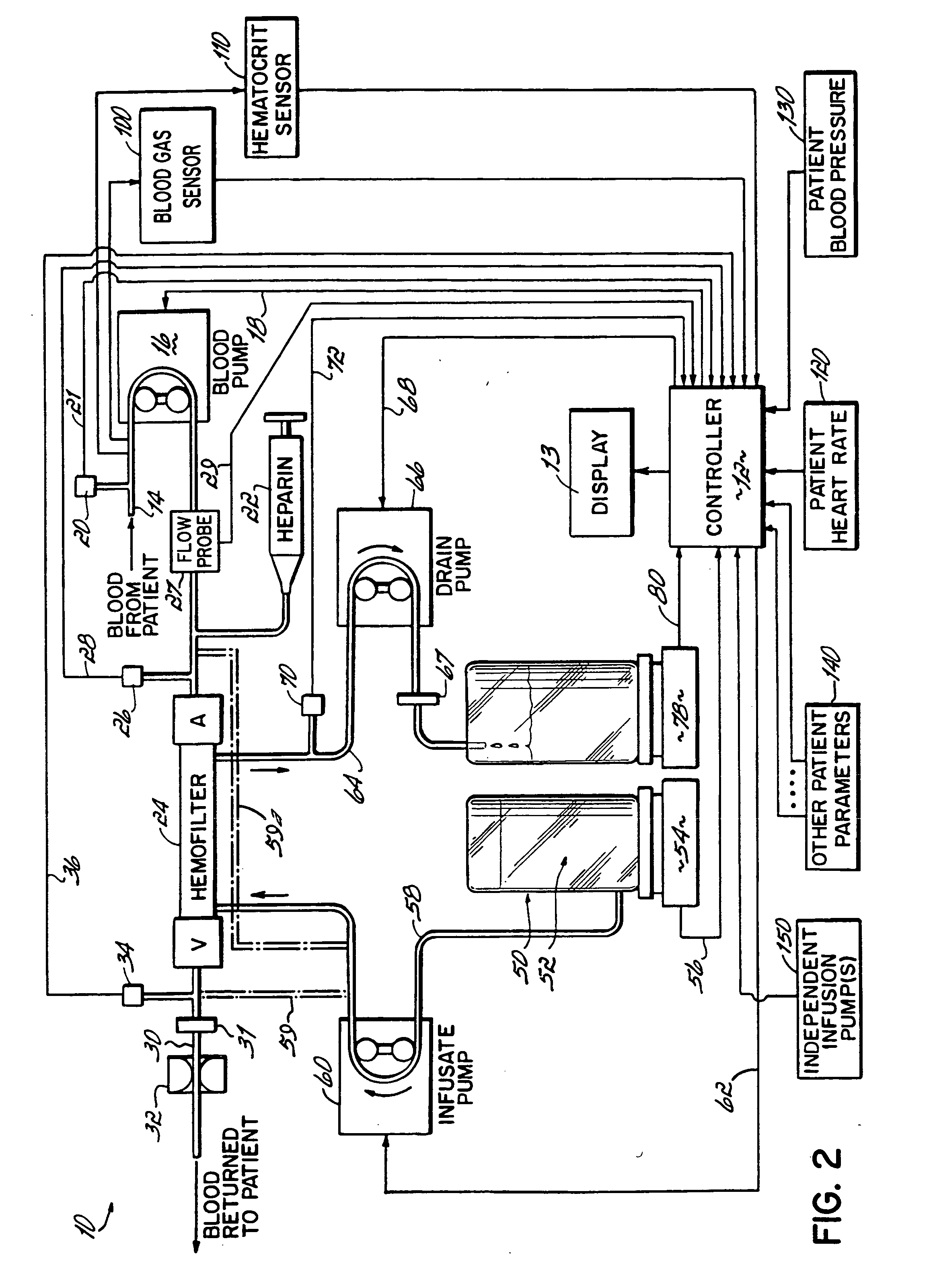
Hemofiltration system and method based on monitored patient parameters, supervisory control of hemofiltration, and adaptive control of pumps for hemofiltration
a hemofiltration system and monitoring patient technology, applied in the field of system and method of blood filtration, can solve the problems of lack of flexibility and accuracy required inability to accurately control the amount of liquids used, and current systems for monitoring and controlling renal replacement procedures do not have the flexibility and accuracy required to perform such procedures on neonates. , the effect of long-term operation, high degree of accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0155] For a ultrafiltration procedure simulated with a container full of blood as the “patient”, the tracking error, the control voltage and the controller parameters are shown in FIGS. 7A, 7B, and 7C, respectively. The tracking error goes to zero within thirty seconds, while the voltage remains steady. A large negative blood pump tracking error at the beginning of the ultrafiltration procedure is tolerated because the patient will not be adversely affected by a slow blood clearance of a few seconds. A large positive tracking error could result in decreased tissue perfusion, and the patient may or may not react adversely to the decrease of nutrients reaching various tissues. Hence, a transiently large negative error at the beginning of the ultrafiltration procedure is tolerated in exchange for preventing tracking overshoot.
example 2
[0156] A ultrafiltration procedure lasting approximately one hour, with the blood flow rate set at 40.0 ml / min, the drain flow rate set to 230.0 ml / hr, and both replacement flow rates set to 100.0 ml / hr simulates an ultrafiltration procedure performed on a neonate. Typical flow rate and weight tracking errors of a simulation utilizing water as a substitute for all fluids are shown in FIGS. 8A, 8B, 8C and 8D. The blood pump tracking error at the beginning of this simulation differs in character from the beginning of the simulation shown in FIGS. 7A-7C because the flowmeter low pass filter choices provided by the manufacturer were not identical for this time period in the two simulations. Given the precise duration of the procedure, the expected ultrafiltration was 30.1 ml. The ultrafiltration measured by comparing the initial (269.3±0.5 gr) and final weight (241.0 ±0.5 gr) of the “patient” was 28.3±0.7 gr, which results in a difference of 1.7±0.7 ml from the desired ultrafiltration. ...
example 3
[0157]FIGS. 9A, 9B, and 9C present a simulation where the scale is bumped twice and a tubing leak occurs. Threshold values for determining an incongruent weight change are given in Table 3.
TABLE 3Minimum No. ofSamples forMaximumValidating AnTrackingMaximumIncongruentPumpErrorPrediction ErrorMeasurement of FlowBlood20 ml / min20 ml / min5Drain 3 gr 3 gr5Replacement 1 3 gr 3 gr5Replacement 2 3 gr 3 gr5
A brief disturbance of a large magnitude is introduced at a n=10 (by placing a large weight on the scale and removing it), and the supervisory controller does not react. At n=60, a similar, smaller disturbance is introduced for a brief period, and again, the supervisory controller does not respond. At n=90, a similar small disturbance is introduced, but for a prolonged period. This simulates a leak in the tubing, and is a much smaller disturbance than is generally encountered when leaks occur during actual ultrafiltration procedures. The controller detects the incongruent weight change an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com