Ultrasound therapeutic device
a therapeutic device and ultrasonic technology, applied in the field of ultrasonic treatment, can solve the problems of manual systems reliant on skills, inconvenient operation, inconvenient adjustment of power, etc., and achieve the effect of maintaining acoustic power and operating safely and effectively
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
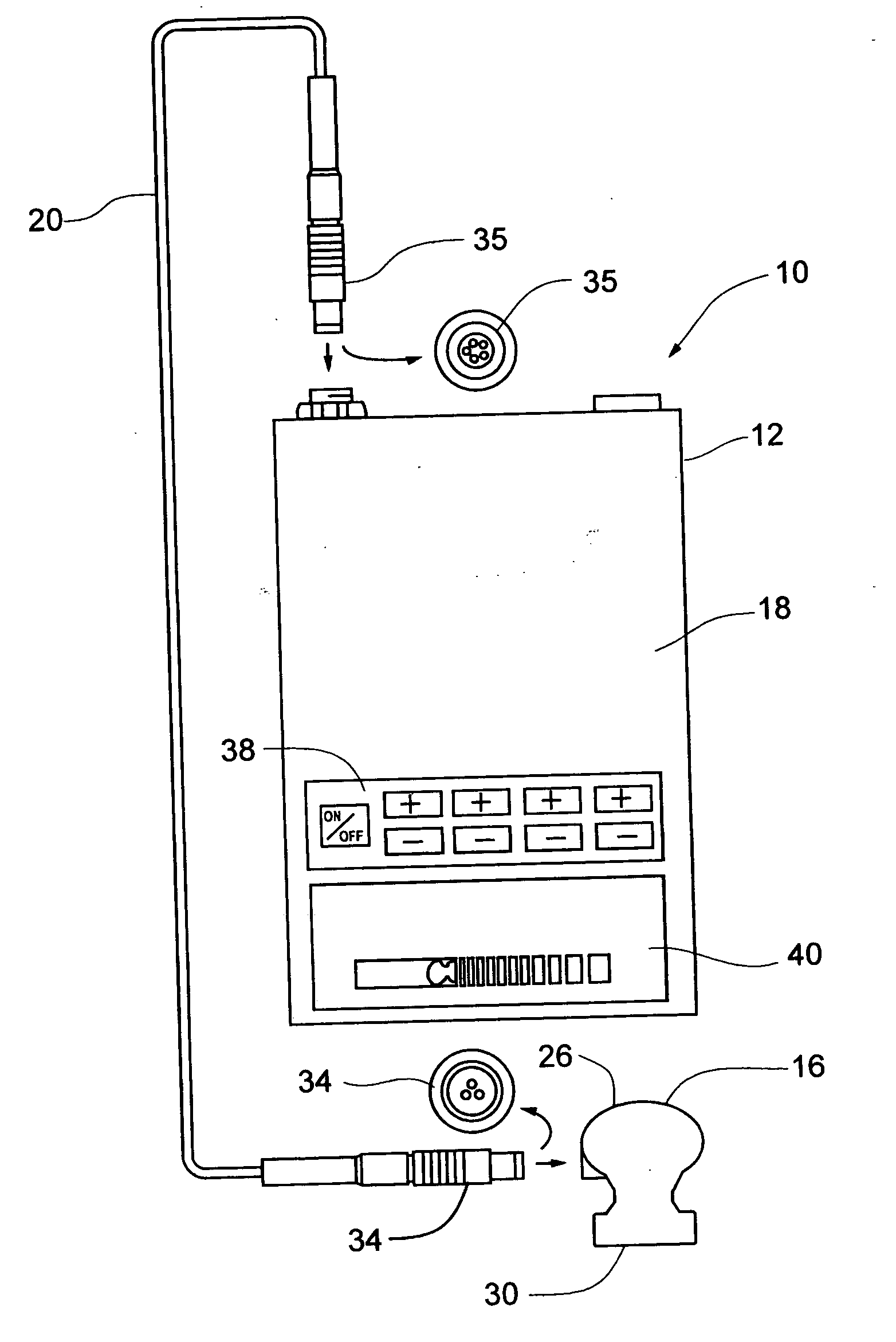
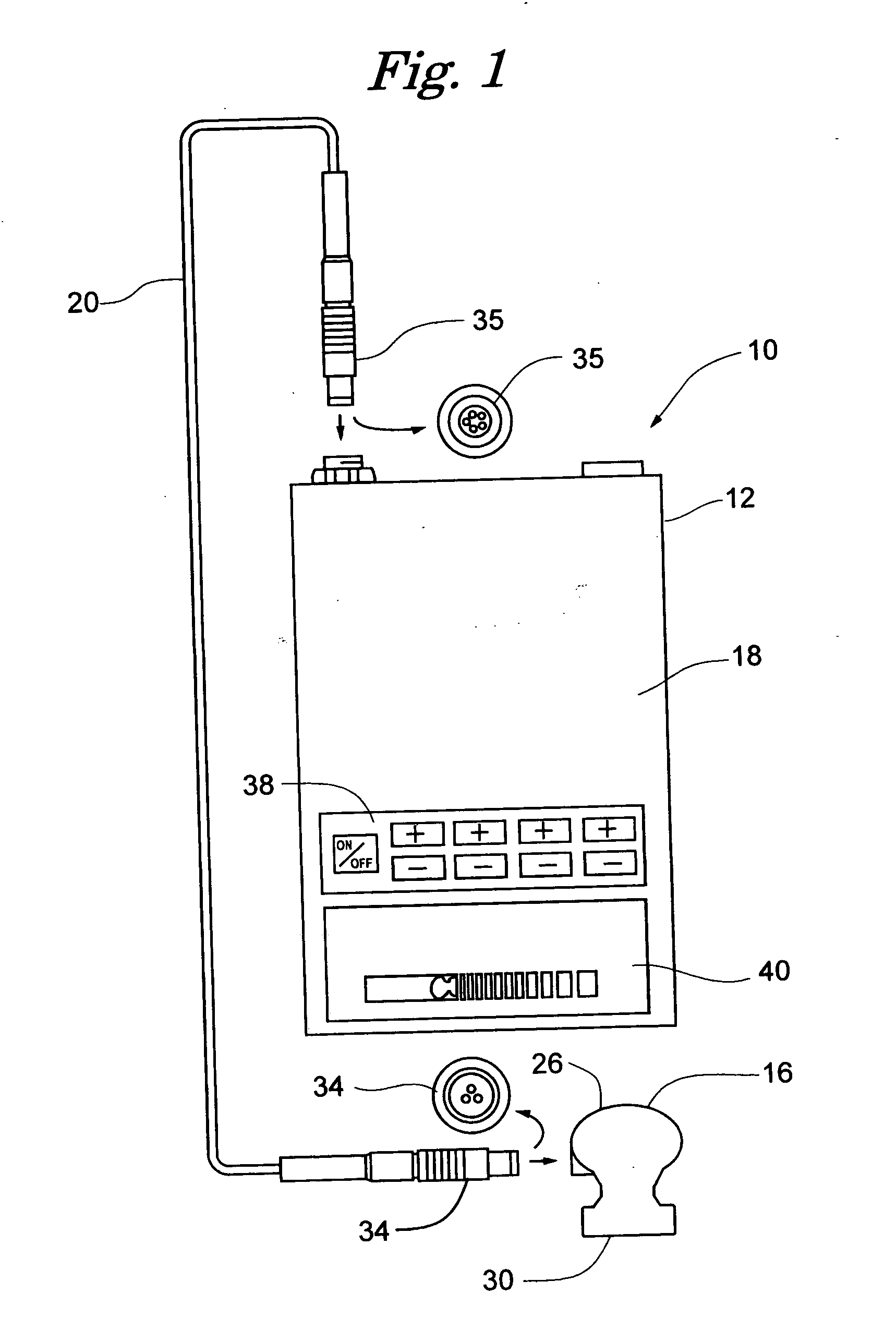
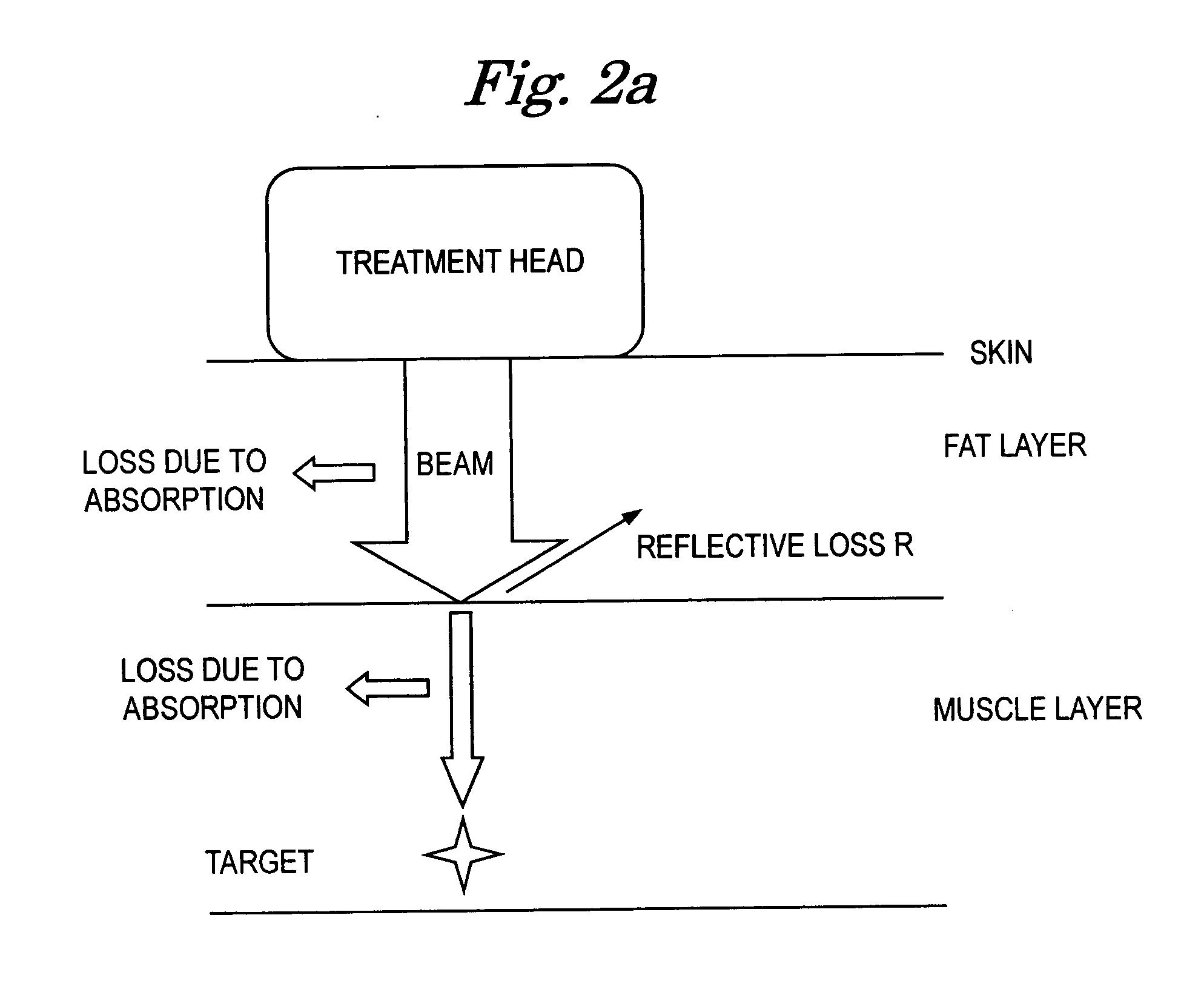
[0031] Referring primarily to FIGS. 1 and 5 , an ultrasound therapeutic system 10 in accordance with the present invention is shown. Specifically, the ultrasound system 10 generally includes a device housing 12 , a generator / output stage high frequency amplifier 14 , at least one transducer treatment head 16 , and a programmable controller 18 . The generator 14 and controller 18 are housed within the device housing 12 , and are both in operable communication with the transducer 6 via a frequency cable 20 .
[0032] The generator 14 , as shown in FIG. 5, is an electric power source commonly understood by one skilled in the art. For instance, a 48 Volt dc, 0.85 Amp main power source can be utilized in one embodiment, while others could implement similar compatible units. First and second working frequencies of the generator 14 are preferably approximately 1.017 MHz and 3.2 MHz, respectively. It is possible to have a measurable tolerance / deviation of + / −30 KHz on the first frequency and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com