One pot synthesis of taxane derivatives and their conversion to paclitaxel and docetaxel
a technology of paclitaxel and docetaxel, which is applied in the field of semisynthesis of taxane derivatives, can solve problems such as structural complexity of taxanes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
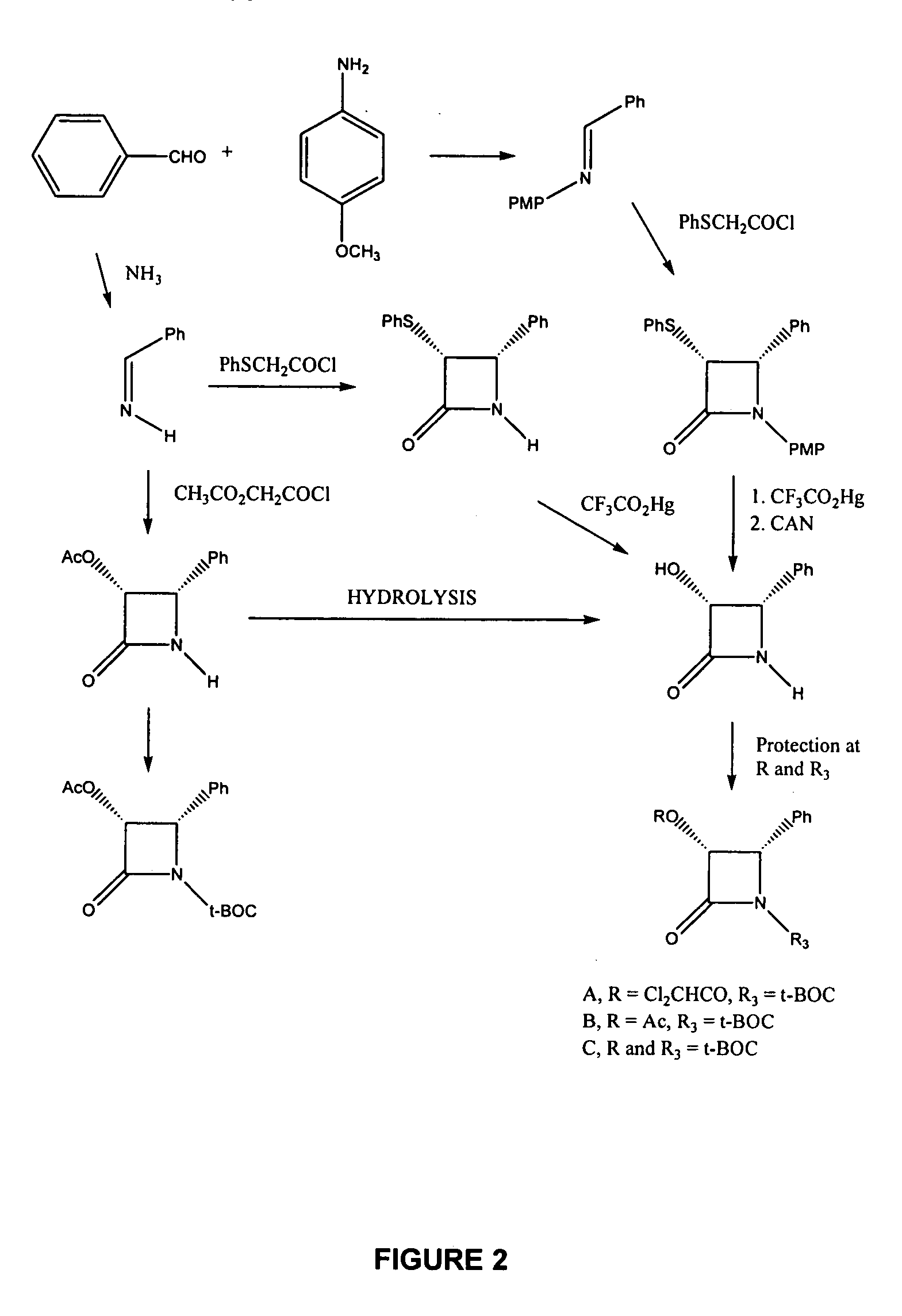
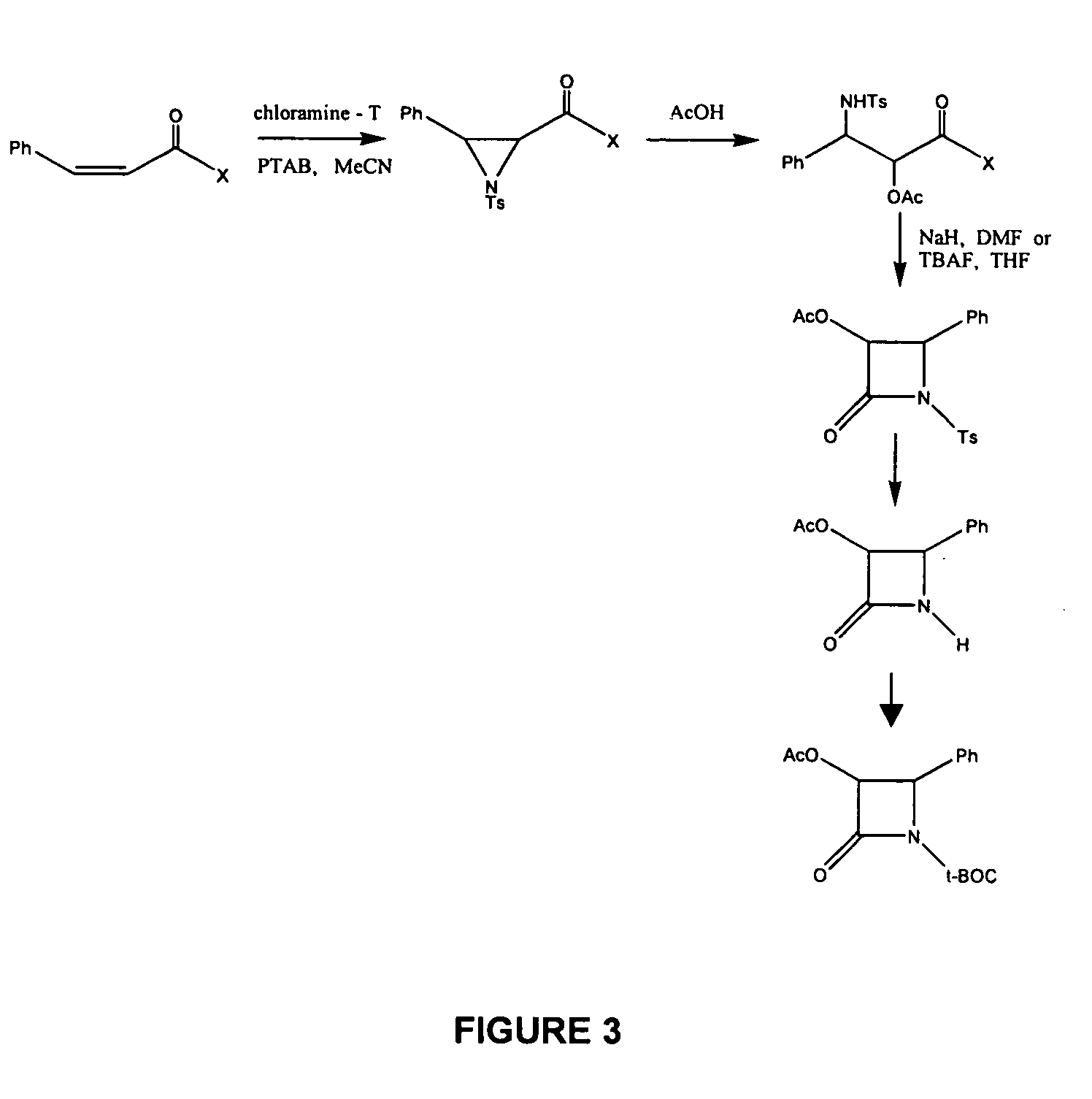
Image
Examples
example 1
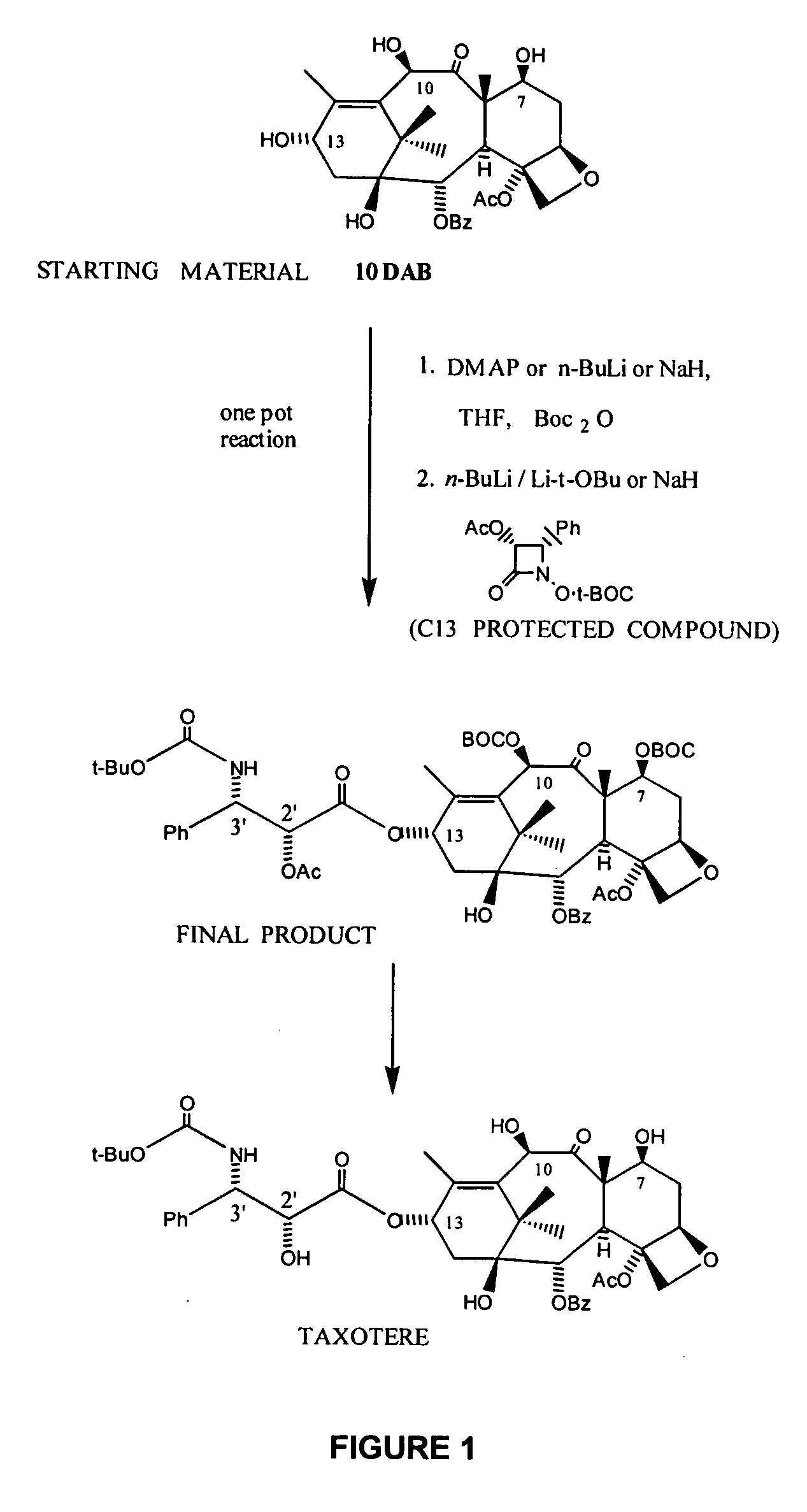
Protection of C-7, 10 Hydroxy Groups and Attachment of a Beta-Lactam Side Chain in a One Pot Reaction
[0116] As shown in FIG. 1, to a stirred solution of 10-deacetylbaccatin III (10-DAB), in an organic solvent, such as THF, at around room temperature under an argon atmosphere was treated with a hydroxy-protecting agent, such as Boc2O, in the presence of a base, such as 4-(N,N-dimethylamino)pyridine or n-BuLi or a mixture of n-BuLi / Li-t-OBu. The reaction was stirred at this temperature for a period between 30 minutes to 2 hours until complete consumption of the starting materials, as evidenced by TLC.
[0117] To this first solution of the C-7,10 protected 10-deacetylbaccatin III derivative in an organic solvent, such as the freshly distilled THF, under argon atmosphere at low temperature most preferably at −40 to −50° C., was added drop wise a solution of a base, such as n-BuLi, in hexanes or a mixture of n-BuLi / Li-t-OBu. After stirring for 30 min to 1 hr at this temperature, a soluti...
example 2
Synthesis of Docetaxel
[0118] As further shown in FIG. 1, the C-13 beta-lactam protected taxane intermediate, was hydrolyzed using formic acid to remove the C-7 and / or C-10 BOC protecting group and then with a mixture of NaHCO3 / Na2CO3 / H2O2 to deprotect the C-2′ and / or C-10 acetate groups to yield docetaxel, as described in U.S. patent application Ser. No. 10 / 790,622, which application is assigned to the assignee of the present invention and is incorporated herein by reference in its entirety.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com