Analgesic compositions containing buprenorphine
a technology of compositions and buprenorphine, which is applied in the field of analgesic compositions, can solve problems such as side effects of buprenorphin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
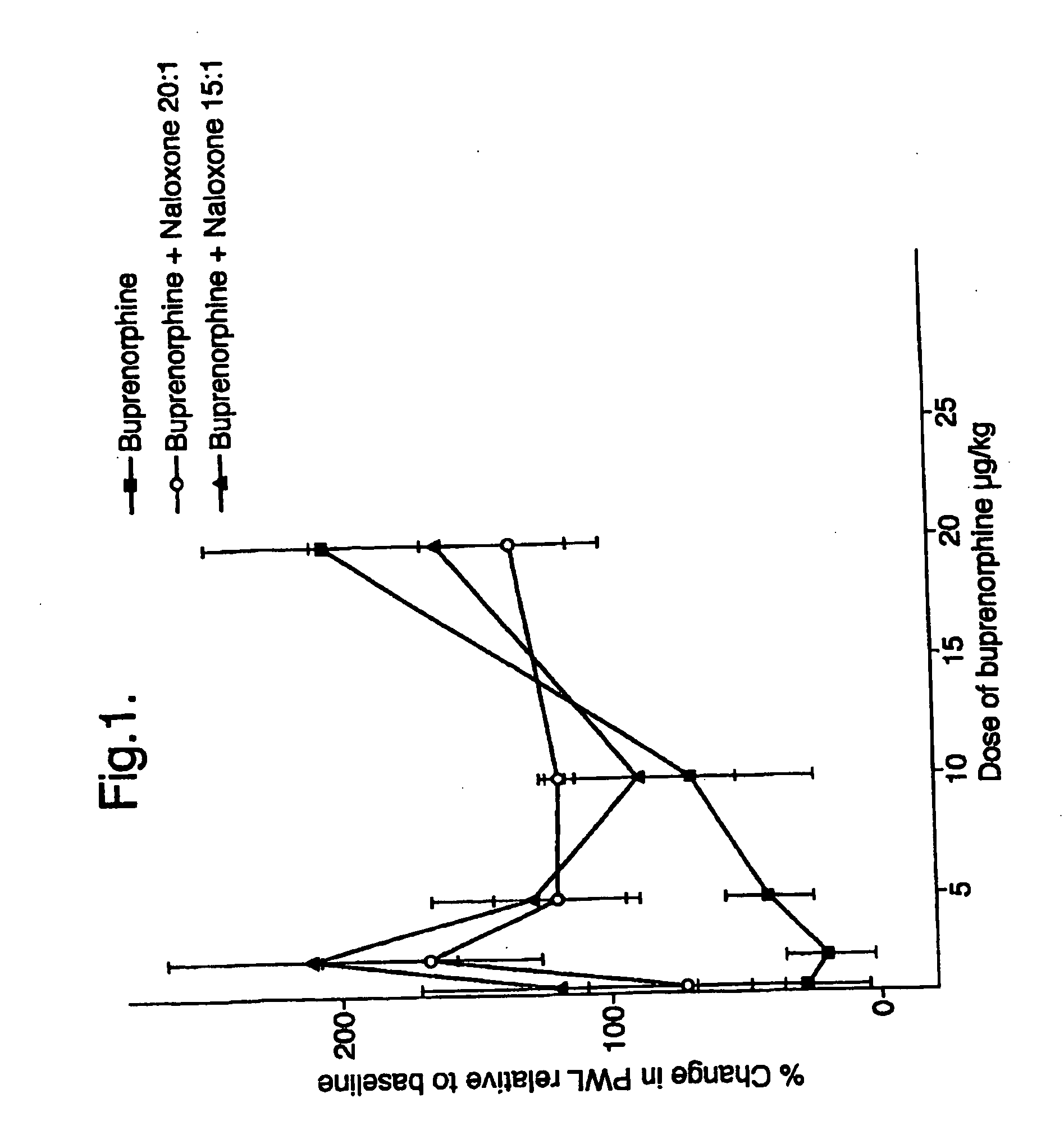
[0045] The effects of buprenorphine and buprenorphine / naloxone at weight ratios of 20:1 and 15:1 were determined at various doses of buprenorphine expressed as μg / kg of body weight of the drug administered subcutaneously to rats (n=3).
[0046] The results of these tests are given in FIG. 1. The potentiation of sub-clinical doses of buprenorphine by low dose naloxone can be clearly seen from the graph of FIG. 1. Both of the buprenorphine / naloxone combinations showed marked increases in pain withdrawal latencies at both weight ratios where the buprenorphine dose was 1.25 μg and 2.5 μg, compared to buprenorphine alone which had no significant effect at these dosage levels.
example 2
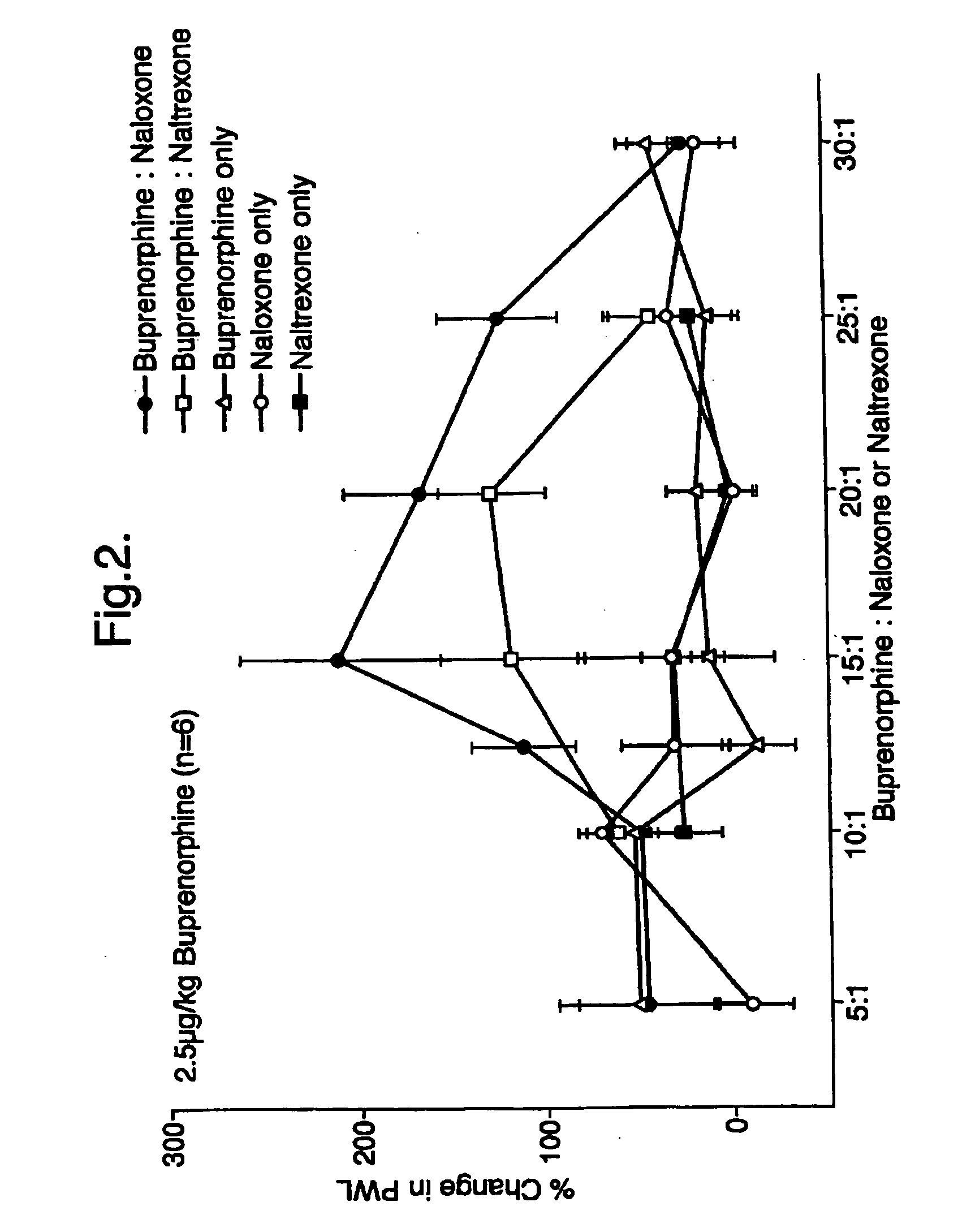
[0047] Buprenorphine was administered to rats (n=6) at a dosage level of 2.5 μg / kg of body weight of the rats. Buprenorphine was co-administered subcutaneously with either naloxone or naltrexone at various weight ratios ranging from 5:1 to 30:1. In order to provide appropriate baseline points naloxone and naltrexone alone were also administered subcutaneously to rats at the same dosage levels as those used in the combined treatments.
[0048] The results are given in FIG. 2 from which the potentiation of the sub-clinical doses of buprenorphine by naloxone or naltrexone can be clearly seen.
example 3
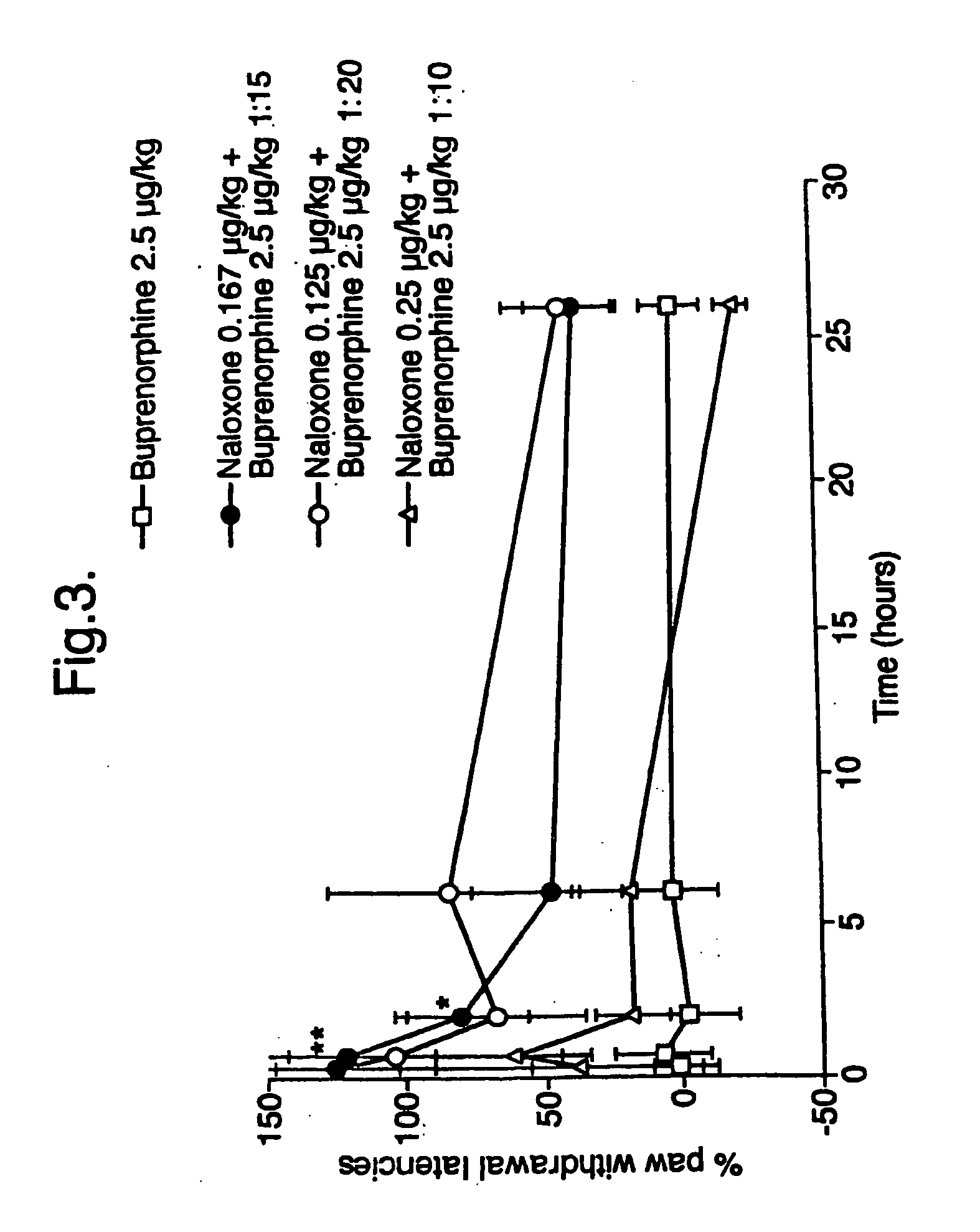
[0049] To investigate the duration of action of several ratios (10:1, 15:1 and 20:1 buprenorphine:naloxone, with a fixed dose of buprenorphine) the effect on PWL was followed over a 26 hour period following subcutaneous injection. The results are given in FIG. 3 from which it can be seen that the effect was already maximal after 40 minutes and then decreased sharply over 6 hours. However at 26 hours a residual effect was still visible, although this was not statistically significant. The maximal effect of each ratio (40 min) was compatible with the results shown in FIG. 2. The effect observed with the 10:1 ratio combination was not statistically significant.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weights | aaaaa | aaaaa |
| weights | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com