Dc-sign blockers and their use for preventing or treating viral infections
a technology of dcsign blockers and viral infections, which is applied in the field of dcsign blockers and their use for preventing or treating viral infections, can solve the problems of no dengue virus vaccine commercially available, hypovolemic shock fatality, etc., and achieve the effects of inhibiting the binding of hiv, inhibiting the binding, and inhibiting the binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Flaviviruses
[0177] The production and purification of DEN type-1 (DEN-1) virus strain FGA / NA d1d (GenBank accession number AF226686) (Duarte dos Santos et al. Virology, 274: 292, 2000) and West Nile (WN) virus strain IS-98-ST1 (GenBank accession number AF481864) (Mashimo et al., PNAS, 99: 11311, 2002) from mosquito Aedes pseudoscutellaris AP61 cell monolayers and virus titration on AP61 cells by focus immunodetection assay (FIA) were performed as previously described (Desprès et al., Virology, 196: 209, 1993). Yellow fever (YF) virus vaccine strain 17D-204 (STAMARIL, Pasteur Vaccins, Lot E113) (GenBank accession number: X07755) was propagated twice in African green monkey kidney VERO cell monolayers, purified in sucrose gradients and titrated on VERO cells. Infectivity titers were expressed as focus forming units (FFU).
[0178] It is noteworthy that FGA / NA d1d E glycoprotein has two N-linked glycosylation sites at positions Asn67 and Asn153. Both N-glycosylation sites of the DEN-1 g...
example 2
Human Mononuclear Cells
[0180] Human DCs from purified mononuclear cells, human monocytic cell line THP-1 (ATCC TIB 202) and DC-SIGN expressing cell clone, THP / DC-SIGN, were obtained from Ali Amara (Immunologie Virale) (Kwon et al., Immunity, 16: 135, 2002). Immature DC, THP-1 and THP / DC-SIGN cells were cultured in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal calf serum (FCS) (Eurobio, lot 160402), 2 mM L-glutamine and antibiotics Peni / Strepto.
[0181] DC were adhered to poly-L-lysine (Sigma)-coated glass Lab-tek chambers (Nalge Nunc International) (5×104 cells per cm2). THP-1 and THP / DC-SIGN cells were adhered to poly-L-lysine-treated glass Lab-tek chambers or polylysine-treated 12-well flasks (5×104 cells per cm2).
example 3
Virus Infections
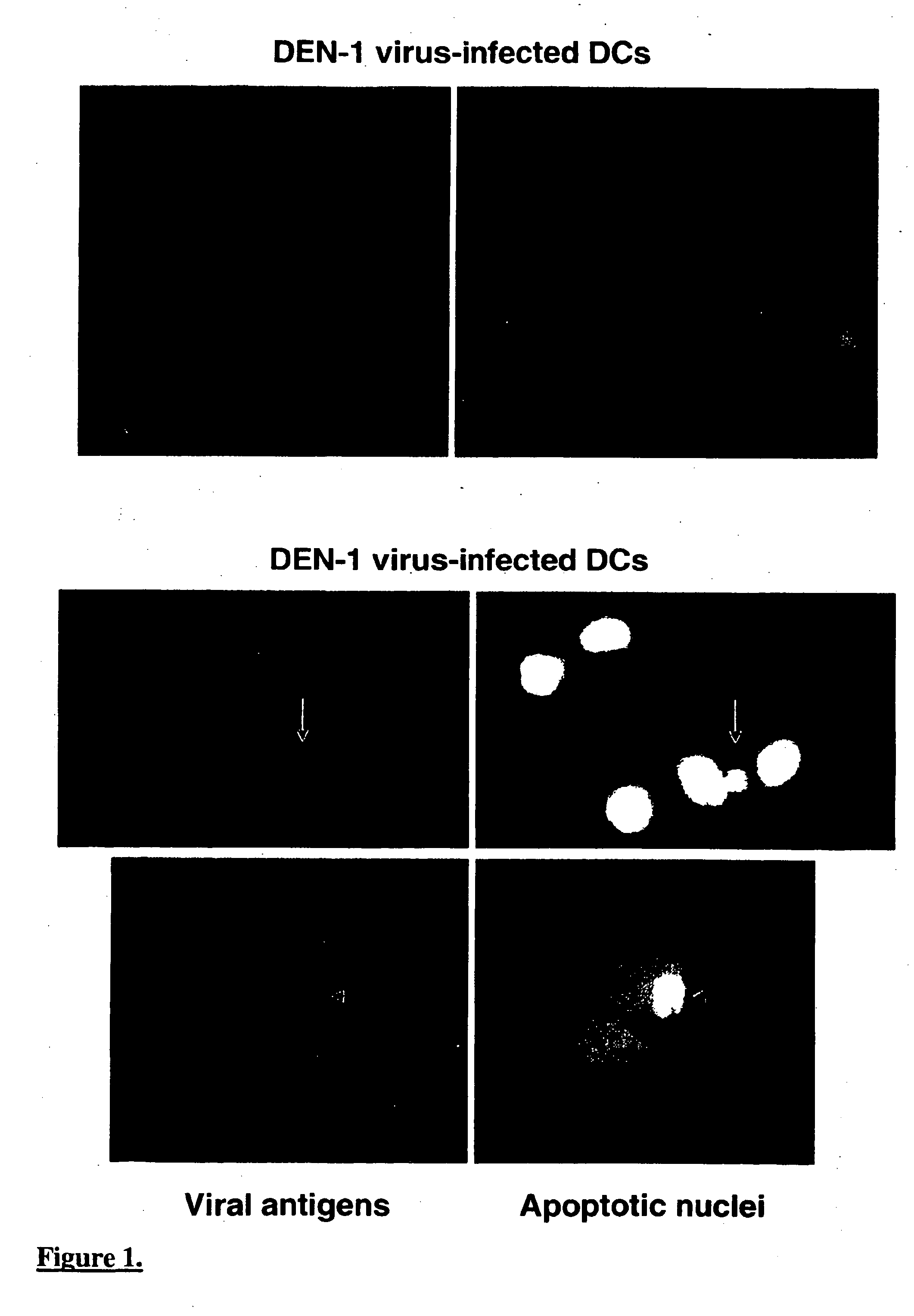
[0182] Cells were washed once with RPMI 1640, incubated with highly purified virus in RPMI 1640 supplemented with 0.2% bovine serum albumine (BSA, pH 7.5) (Sigma) for 2 hrs at 37° C. and placed into fresh media supplemented with 2% FCS, 2 mM L-glutamine and antibiotics Peni / Strepto at 37° C. for 40 hrs.
[0183] The percentage of cells expressing viral antigen was determined using either DEN-1 virus-specific hyperimmune mouse ascites fluid (HMAF) 9801 (strain Hawaï), WN virus-specific HMAF 0801 (strain IS-98-ST1), or YF virus-specific HMAF 9803 (strain FNV) with dilution of 1:50, by indirect immunofluorescence as previously described (Desprès et al., J.Virol., 70: 4090, 1996).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap