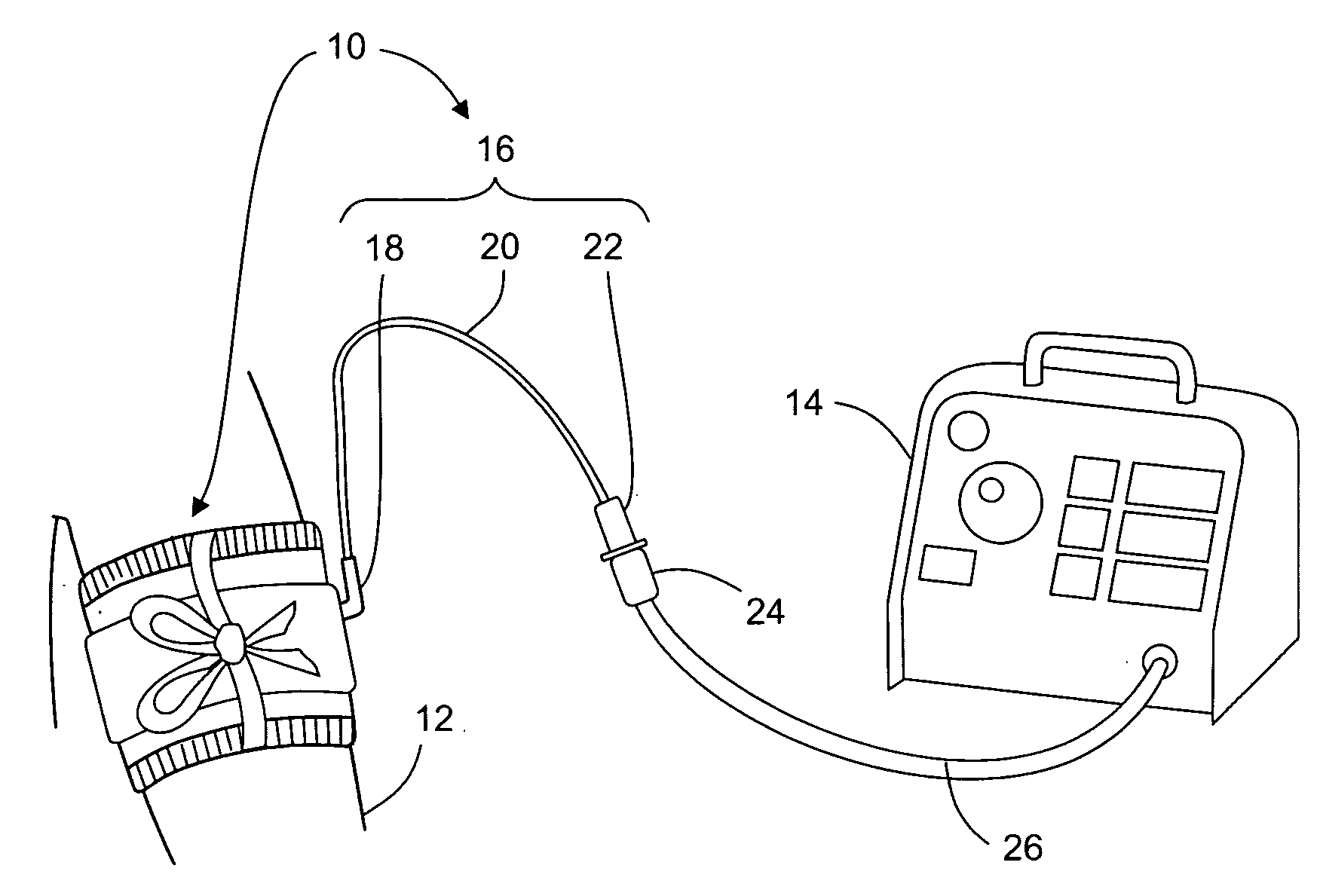
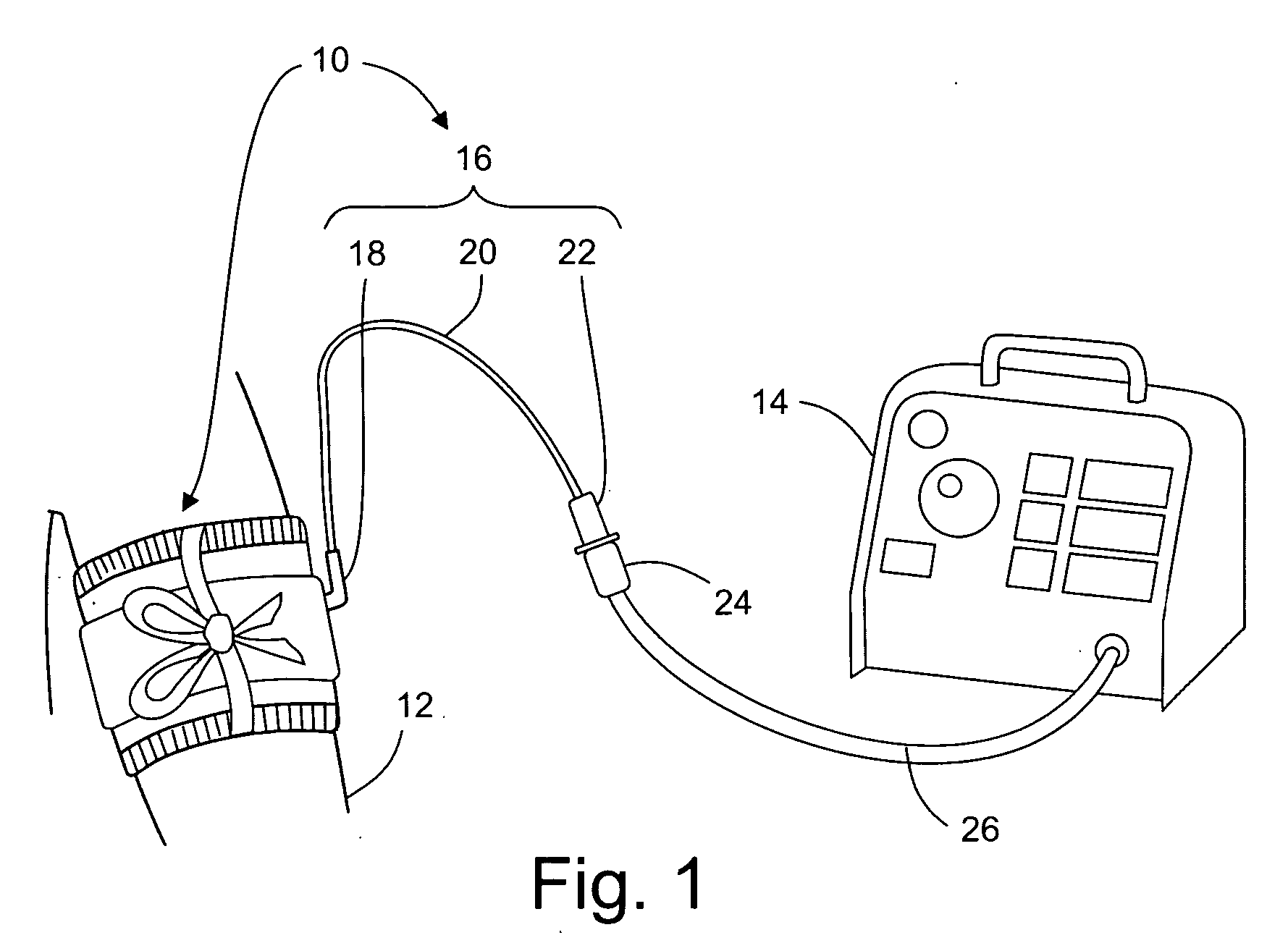
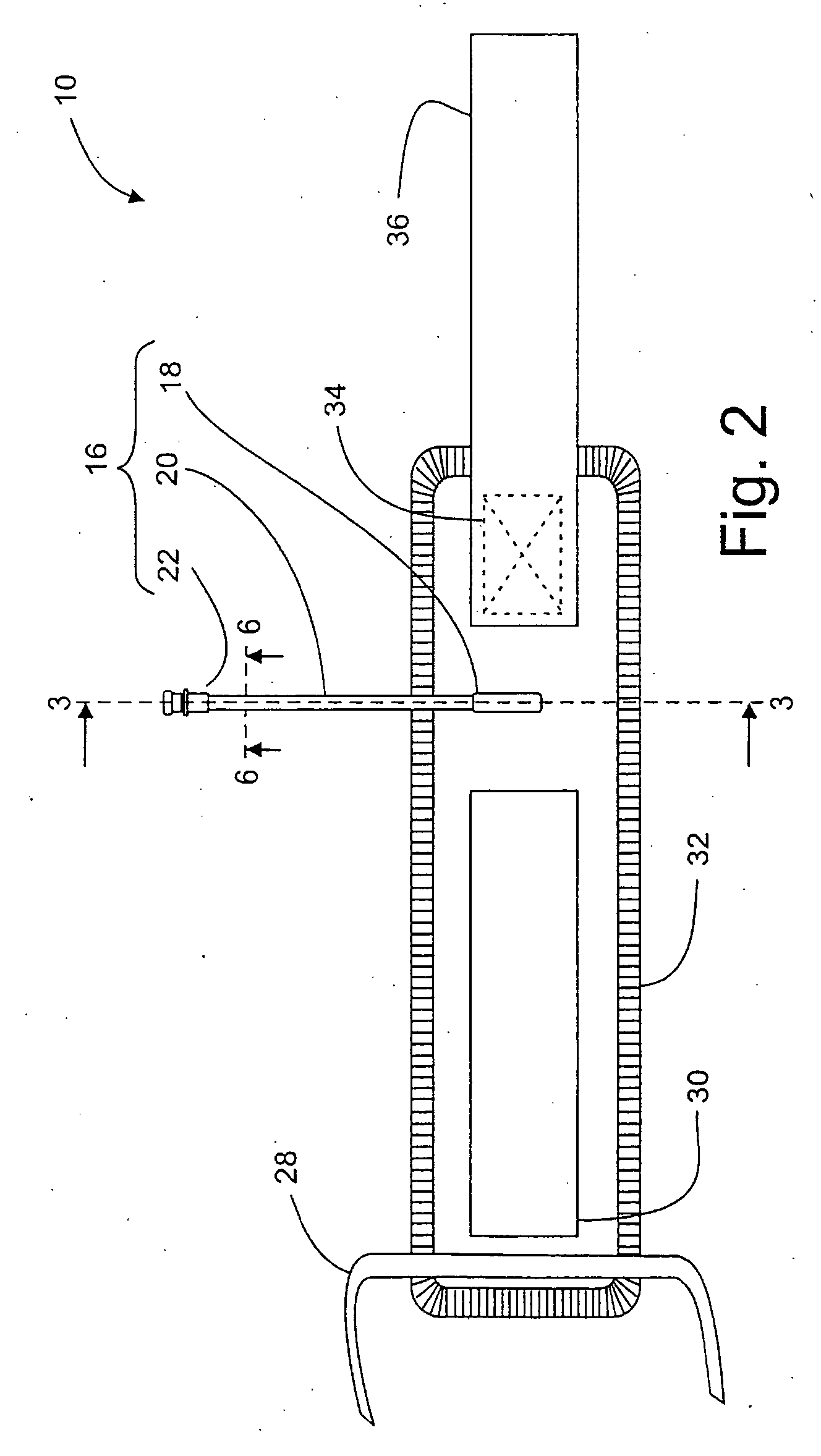
One
hazard associated with all pneumatic tourniquet cuffs of the prior art is the obstruction of the pneumatic passageway within the cuff.
Any isolated region may be hazardous, either by permitting
arterial blood to flow into the limb past a region of lower
cuff pressure or by requiring surgical staff to set the tourniquet instrument to an unnecessarily
high pressure to stop
blood flow past the cuff.
Also, any complete obstruction of the pneumatic passageway within a tourniquet cuff of the prior art may render ineffective any audio-visual safety alarms of a connected prior-art tourniquet instrument intended to warn of hazardous over-pressurization or under-pressurization of the cuff, such as the safety alarms described by McEwen in U.S. Pat. No. 4,469,099.
A
partial obstruction of the pneumatic passageway at the port connector, or elsewhere within the port or inflatable portion of a prior-art cuff may increase the pneumatic flow resistance at the
partial obstruction, and thus may affect the ability of a connected tourniquet instrument to rapidly and accurately regulate pressure past the partial obstruction and throughout the inflatable portion of the tourniquet cuff.
Increased flow resistance from a partial obstruction may also reduce the ability of a connected tourniquet instrument to accurately and rapidly indicate changes of the pressure in the tourniquet cuff to surgical staff.
Further, a partial obstruction of the pneumatic passageway within a region of the inflatable portion of the cuff may affect the ability of the tourniquet instrument to uniformly regulate pressure throughout the entire inflatable portion of the cuff.
In addition to the hazards of complete and partial obstructions that may affect the integrity of the pneumatic passageway, another
hazard associated with prior-art cuffs is the interruption of the passageway due to unanticipated detachment of the port connector from the tourniquet instrument, or detachment of the port connector from the port, thus separating the inflatable portion of the tourniquet cuff from the tourniquet instrument.
A related hazard is a leak at the port connector that is sufficiently large to prevent a connected tourniquet instrument from maintaining
cuff pressure near the desired pressure.
Such a large leak may result, for example, from deterioration or deformation of the connector of a single-use disposable tourniquet cuff as a result of reprocessing and reuse of the disposable tourniquet cuff in multiple
surgical procedures in a manner neither intended nor anticipated by the manufacturer.
Although such-long and flexible port tubing facilitates connection of the port to non-sterile instrument tubing away from the sterile surgical field, it may also increase the possibility of partial or complete obstruction of the pneumatic passageway within the port, for example by accidental kinking, bending, or pinching of the tubing.
Reprocessing, cleaning and re-sterilizing of disposable tourniquet cuffs may result in hazards for the
surgical patients on whom such cuffs are subsequently used.
The hazard arises from the use of any of a variety of chemical or physical agents that are attendant with the reprocessing, cleaning or re-sterilizing processes.
Exposure of prior-art cuffs to sterilizing agents different than the sterilizing agent employed at the time of manufacture may produce a change and deterioration in the properties of some cuff materials and components, for example due to a
chemical reaction or
exposure to
radiation.
Exposure of a prior-art cuff containing flexible
thermoplastic materials to an elevated temperature during cleaning or sterilization by known prior-art processes may soften
thermoplastic materials and components, increasing the likelihood of hazardous deformation of some components.
For example, an elevated temperature during reprocessing may result in substantial deformation of the
thermoplastic stiffener included in some prior-art cuffs, thus impairing the application of pressure by such a cuff to an underlying limb upon subsequent use in
surgery.
Also, an elevated temperature during reprocessing may deform the thermoplastic connectors of some prior-art cuffs, or may weaken the retention force of typical thermoplastic barb-type port connectors, so that such connectors cannot establish or reliably maintain a gas-tight passageway between the tourniquet cuff and tourniquet instrument upon reuse.
An elevated temperature associated with cleaning or re-sterilization increases the likelihood that the pneumatic passageway within the cuff may become partially or completely obstructed, as described above, as a result of such reprocessing.
Repeated cleaning, re-sterilization and reuse of a disposable tourniquet cuff in multiple
surgical procedures may progressively increase the hazard for the
surgical patients on whom the cuff is used.
 Login to View More
Login to View More  Login to View More
Login to View More