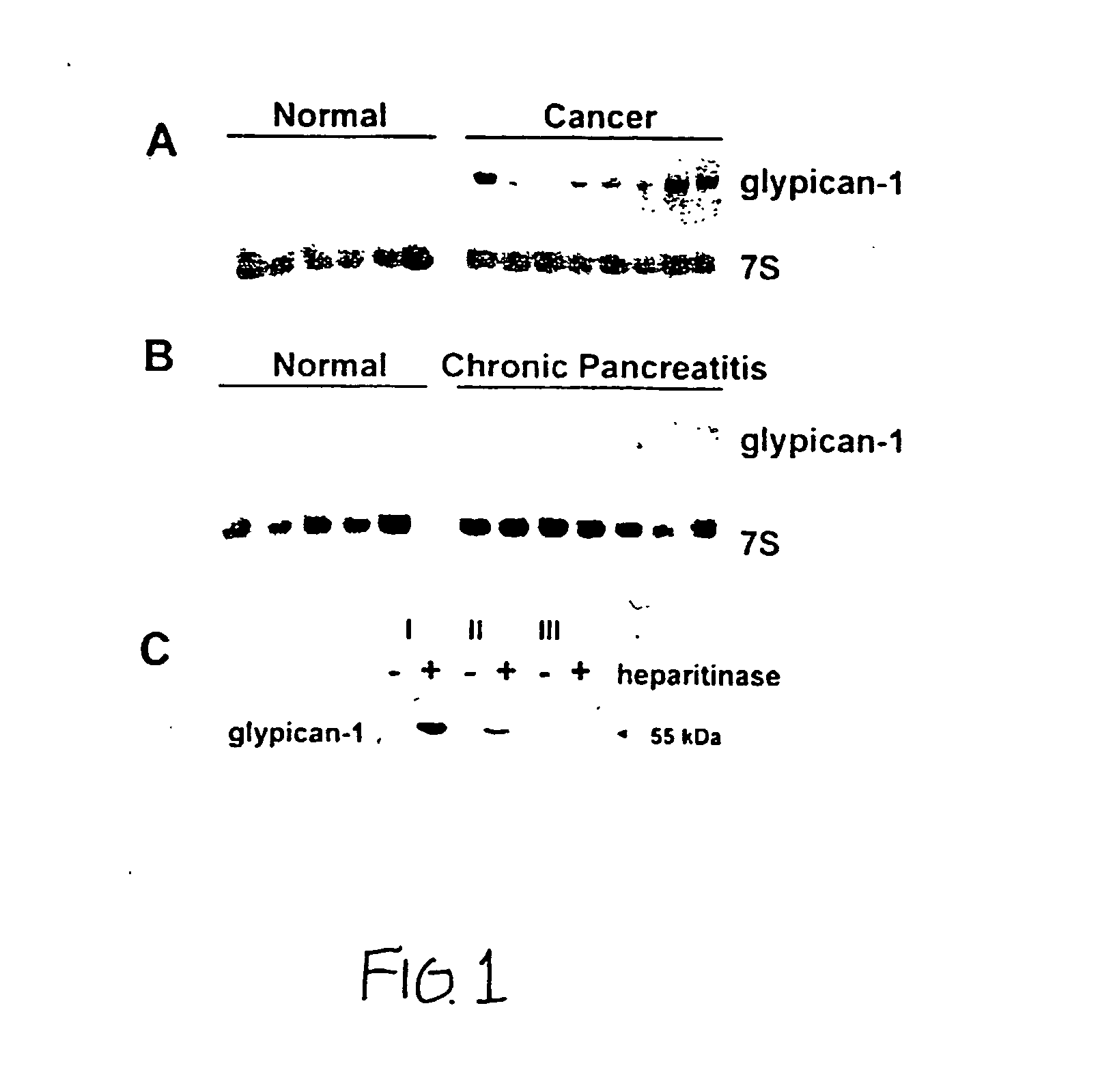
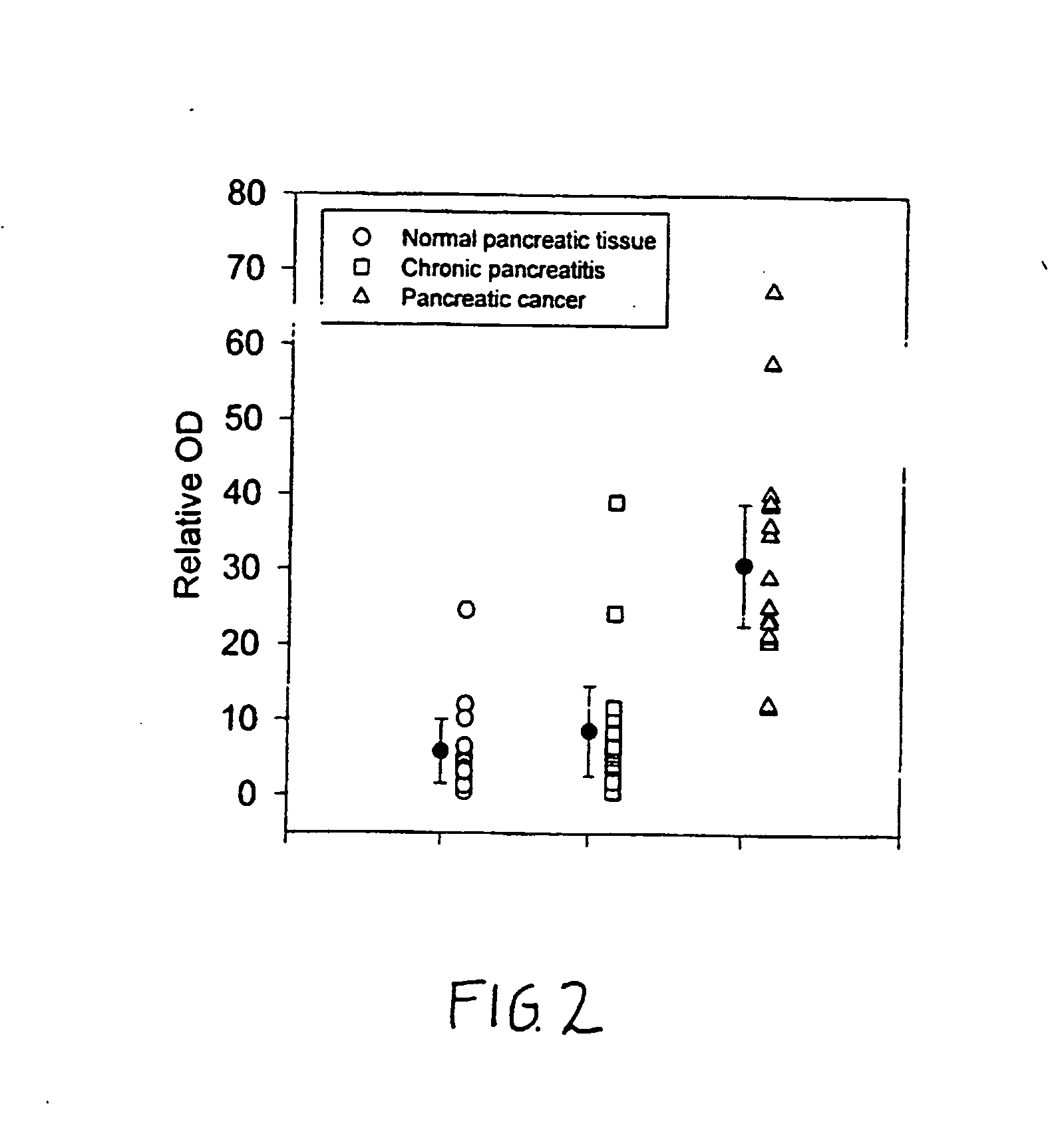
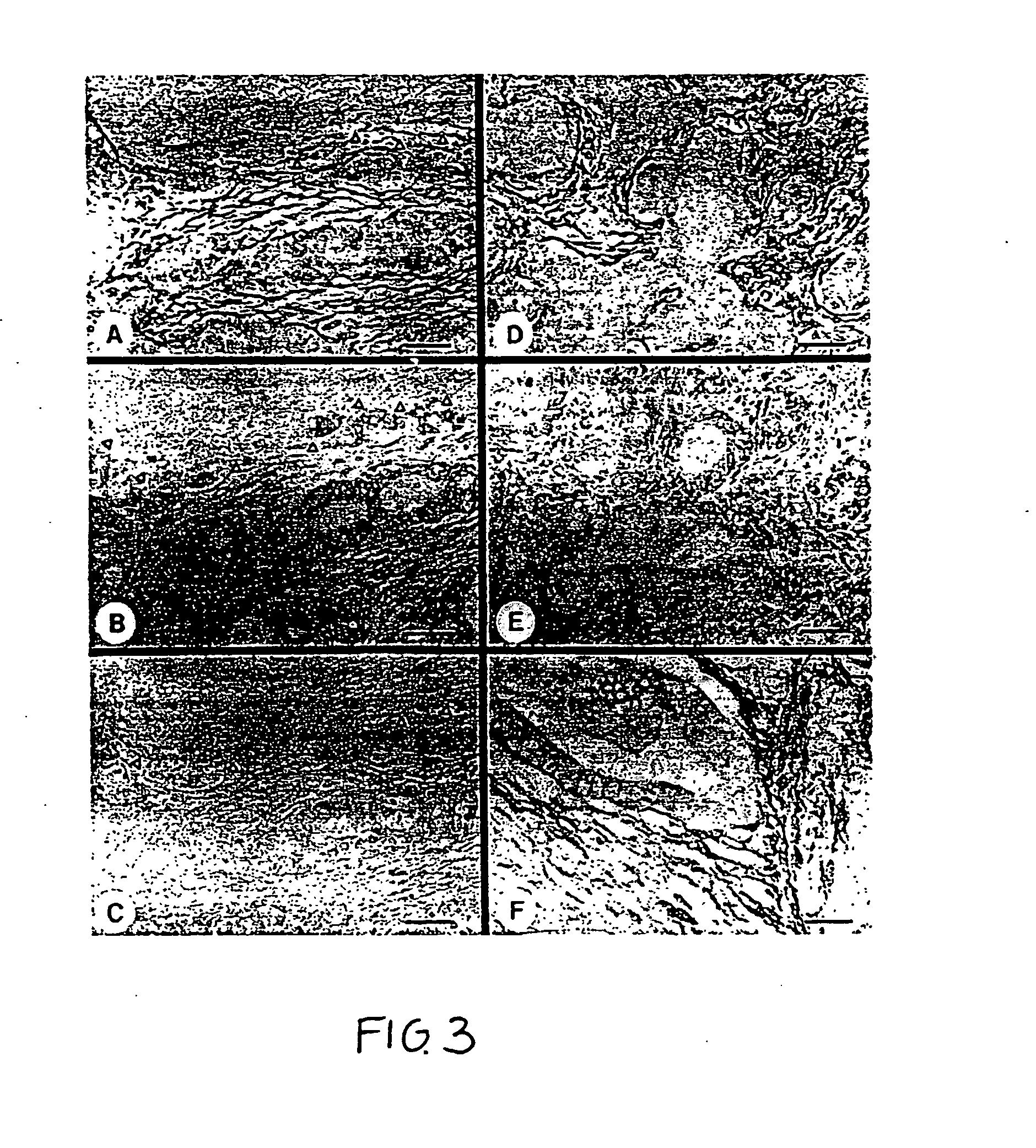
Glypican-1 in human breast cancer
a technology of glypican and breast cancer, applied in the field of medical sciences, to achieve the effect of reducing syndecan-1 levels and retarding the growth of glypican-responsive cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0052] The following description is provided to enable any person skilled in the art to make and use the invention and sets forth the best modes contemplated by the inventor of carrying out his invention. Various modifications, however, will remain readily apparent to those skilled in the art, since the general principles of the present invention have been defined herein specifically to provide an inventive use of glypican-1 and agents binding to and suppressing expression of glypican-1 for detection and therapy of human carcinomas.
(1) MATERIALS AND METHODS
Materials
[0053] The following materials were purchased: FBS, DMEM and RPMI medium Leibovitz's medium, trypsin solution, penicillin-streptomycin solution, and Geneticin (G418) from Irvine Scientific (Santa Ana, Calif.); Genescreen membranes from New England Nuclear (Boston, Mass.); restriction enzymes, pMH6 vector, the random primed labeling kit, the Genius 3 non-radioactive nucleic acid detection kit, and the Genius 4 RNA lab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distance | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com