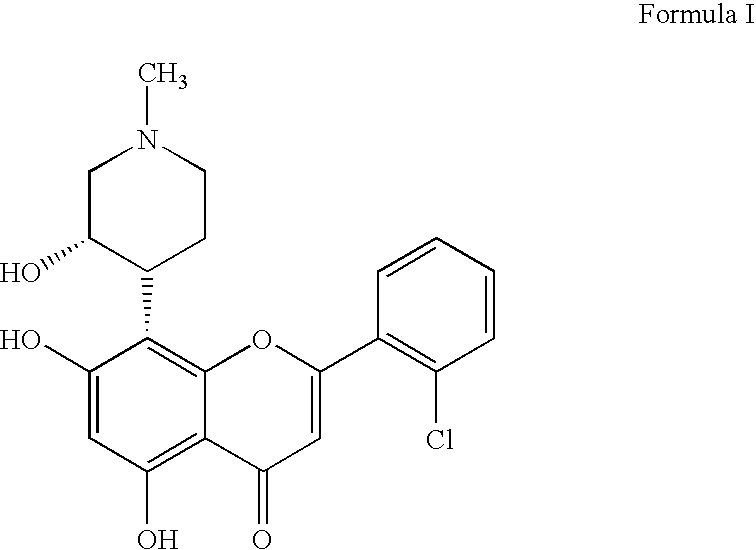
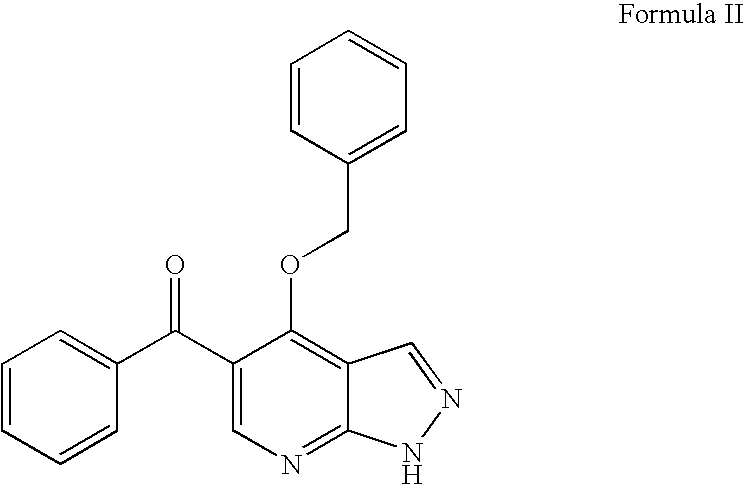
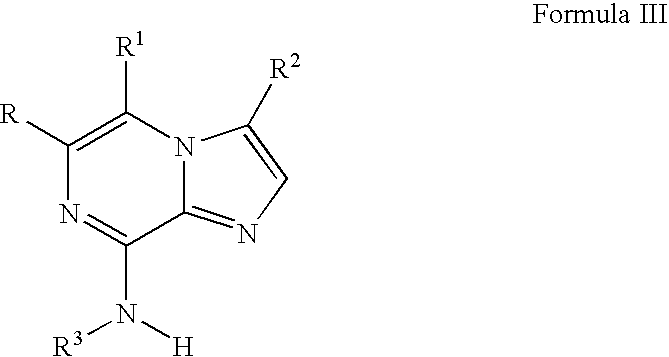
Novel imidazopyrazines as cyclin dependent kinase inhibitors
a cyclin-dependent kinase inhibitor and imidazopyrazines technology, applied in the field ofimidazo1, can solve the problems of increased cdk2 activity and poor overall survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparative Example 1
[0140]
[0141] A mixture of 1-methylimidazole-2-carboxamide (1 equiv.) and bromoacetone (1.2 eq) in anhydrous CH3CN is stirred and refluxed under N2 for 1 day. The solvent is evaporated and the pure product is obtained after column chromatography on silica gel with CH2Cl2:7 N NH3 in MeOH.
examples 2-5
Preparative Examples 2-5
[0142]
[0143] These compounds are prepared by essentially same procedure set forth in Preparative Example 1.
example 6
Preparative Example 6
[0144]
[0145] A mixture of the product from Preparative Example 1 (1 eq.) and imidazole (25 eq.) is stirred under N2 at 175° C. for 20 hr. The imidazole is distilled off in a vacuum and the residue is purified by column chromatography on silica gel with CH2Cl2:7 N NH3 in MeOH. Pure product is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com