Identification of self and non-self antigens implicated in autoimmune disease
a technology of autoimmune disease and identification of self and non-self antigens, applied in the field of immunology, can solve the problems of not using such alignments, no evidence, no evidence, etc., and it is more difficult to define mhc class ii binding motifs based on sequence alignments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
1. Identification of Self Epitopes of Pemphigus Vulgaris
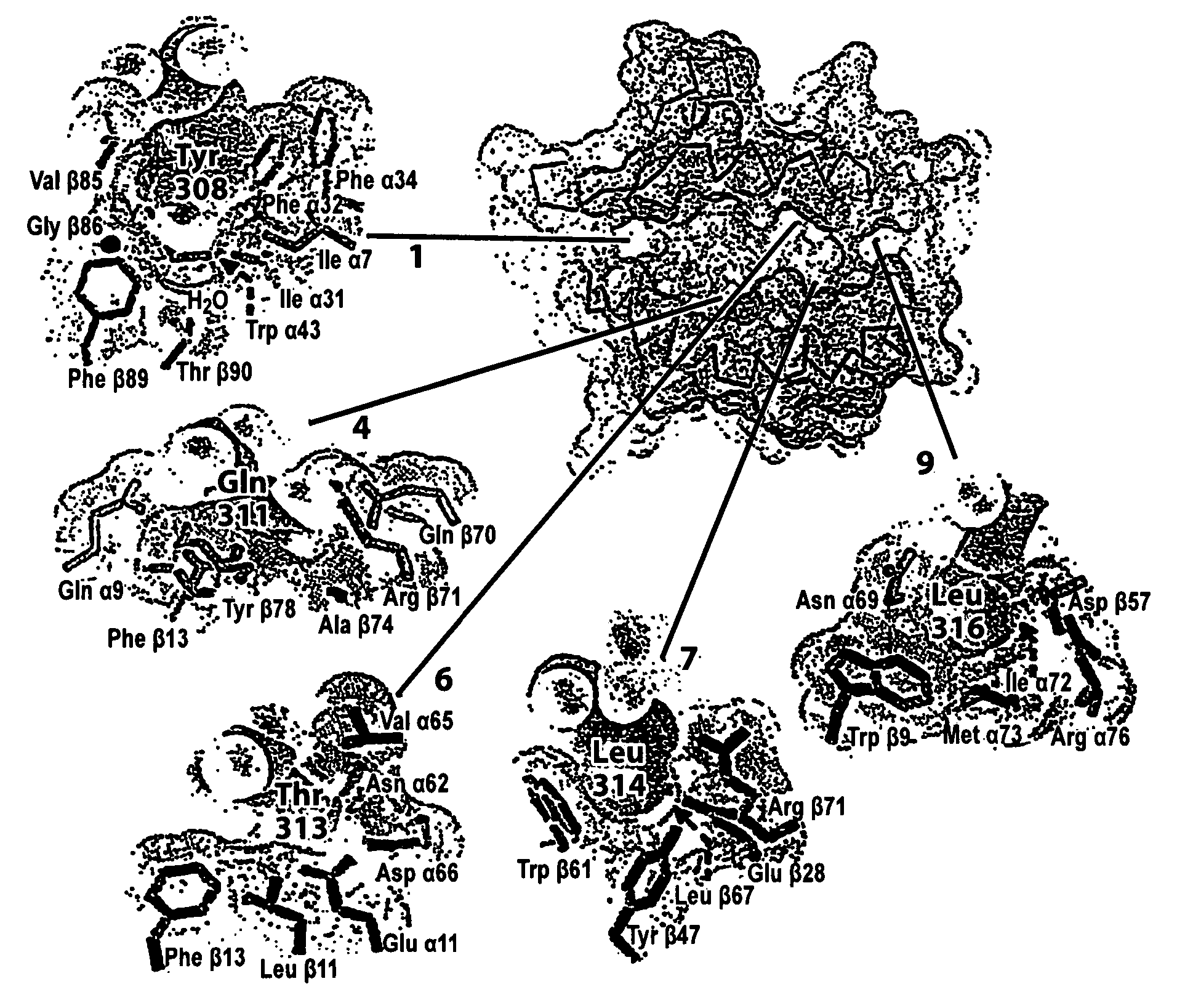
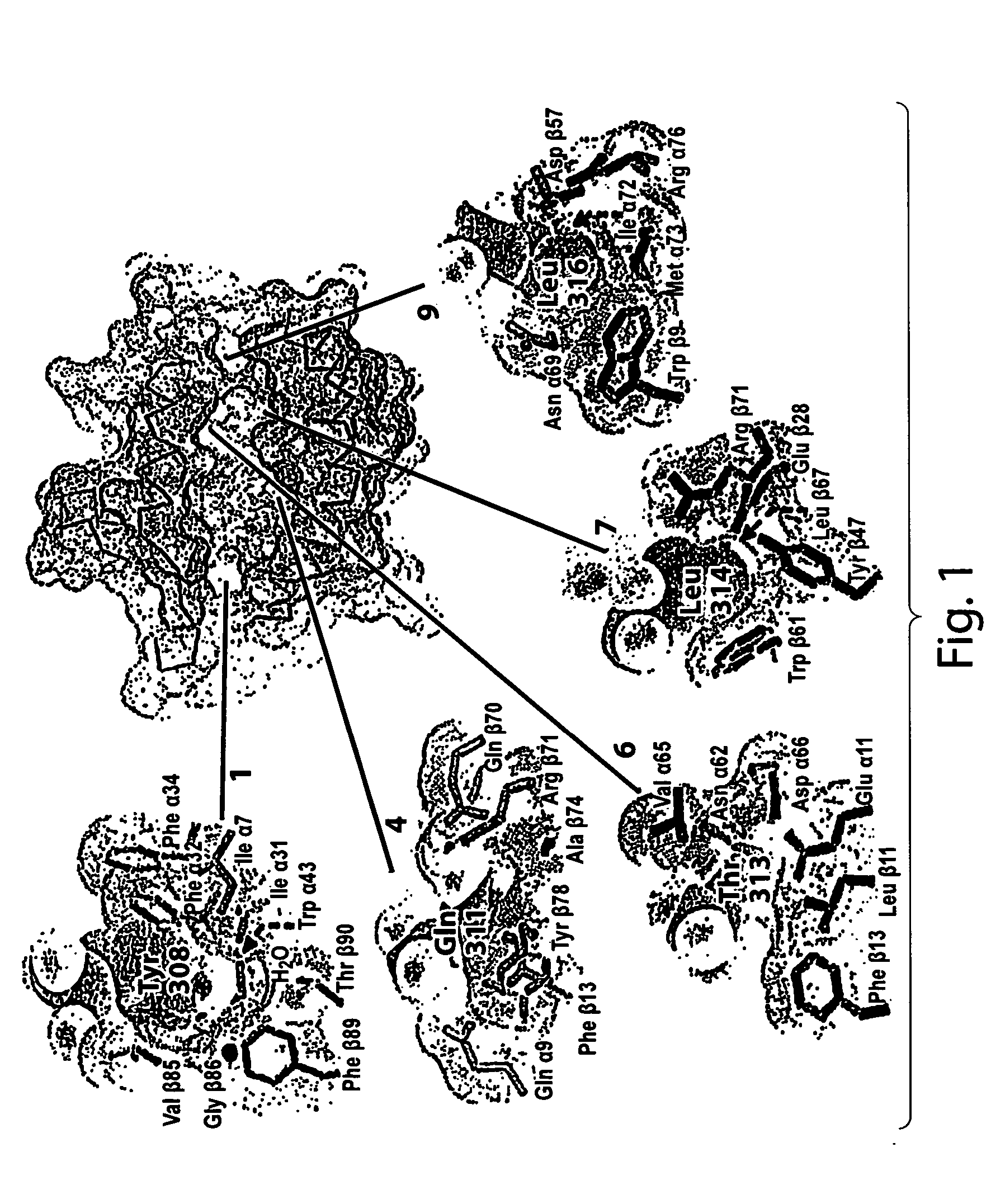
[0090] As noted above, pemphigus vulgaris (PV) is, in different ethnic groups, associated either with a DR4 allele (DRB1*0402) or with a rare DQ1 allele (DQB1*05032); only a small fraction of PV patients have neither susceptibility gene (Ahmed et al., 1991; Ahmed et al., 1990; Scharf et al., 1988b). The PV associated molecule has a negative charge (Glu) at the critical position β71; the neighboring position (β70) is also negatively charged. The DR4 subtype associated with PV is the only one that carries a negative charge at DRβ 71 (a positive charge (Arg) is found at DRβ 71 in the RA associated DR4 molecules). Although polymorphic, the P7 pocket residue DRβ 67 (Leu / Ile) does not appear to be involved in peptide binding but probably acts as a TCR contact residue (Stern et al., 1994).
[0091] The charge of a polymorphic residue at DRβ 71 could therefore account for susceptibility to two different autoimmune syndromes associated w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angstrom probe radius | aaaaa | aaaaa |
| non-myelin basic | aaaaa | aaaaa |
| acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com