Agonists of a2a adenosine receptors for treating recurrent tumor growth in the liver following resection
a technology of adenosine receptor and liver, which is applied in the direction of drug composition, biocide, heterocyclic compound active ingredients, etc., can solve the problems of unknown mechanisms underlying the accelerated outgrowth of inflammation-associated late-stage metastatic tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
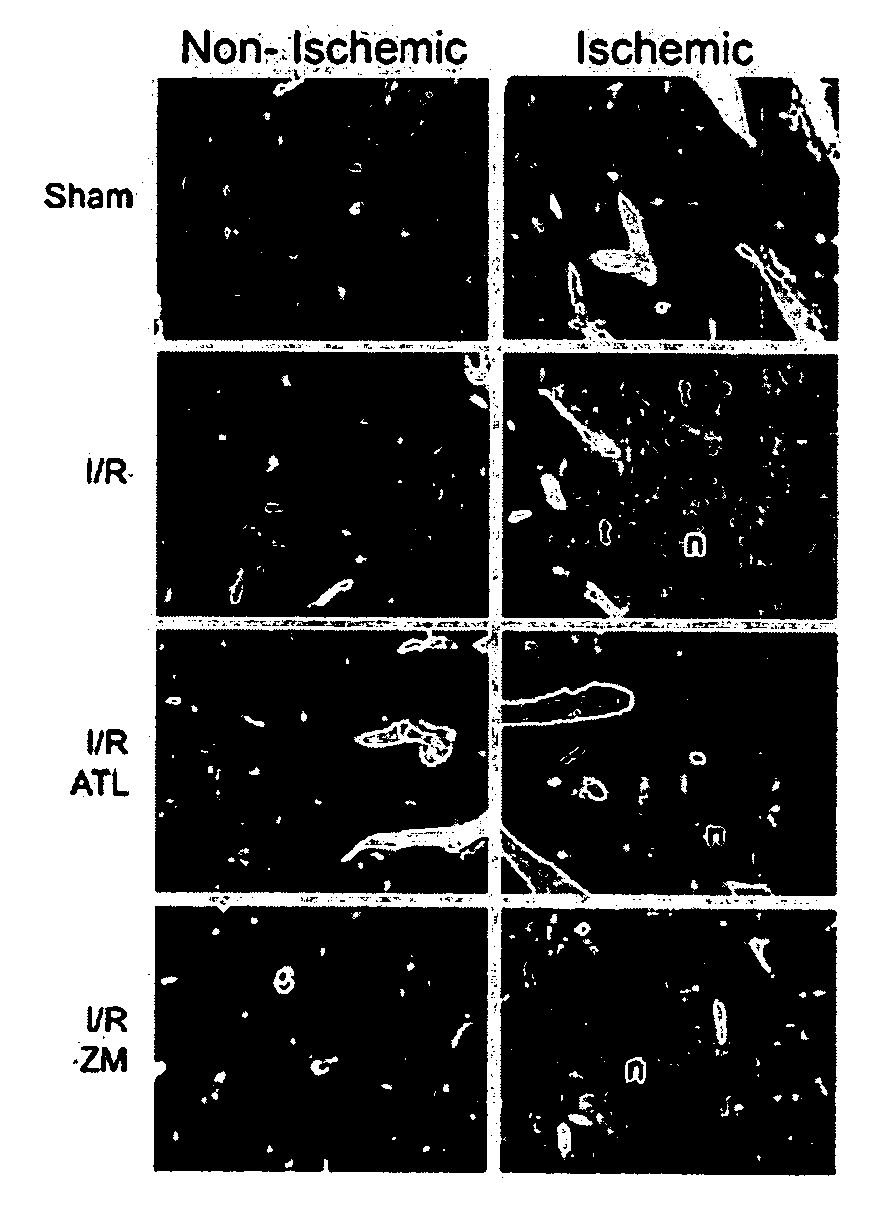
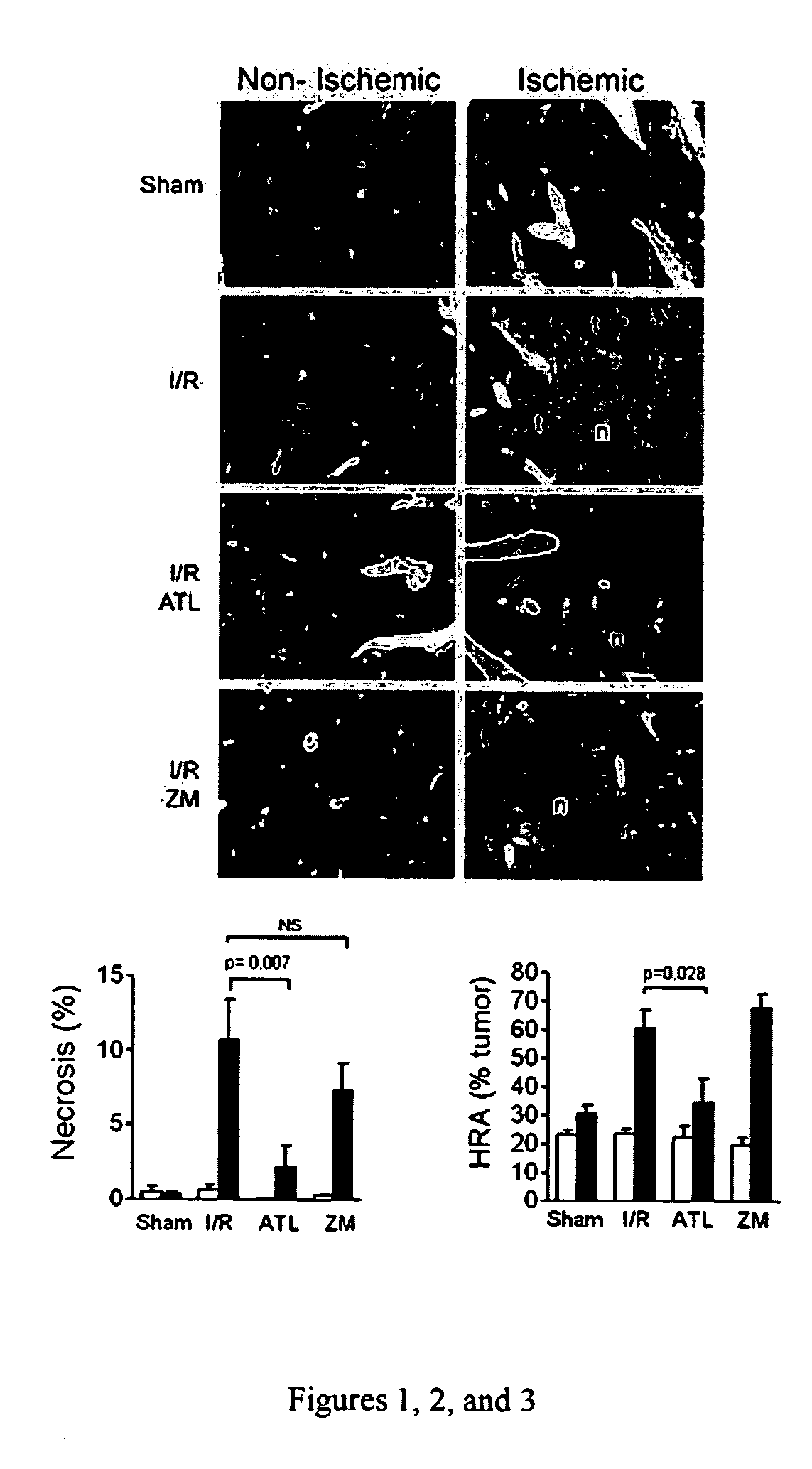
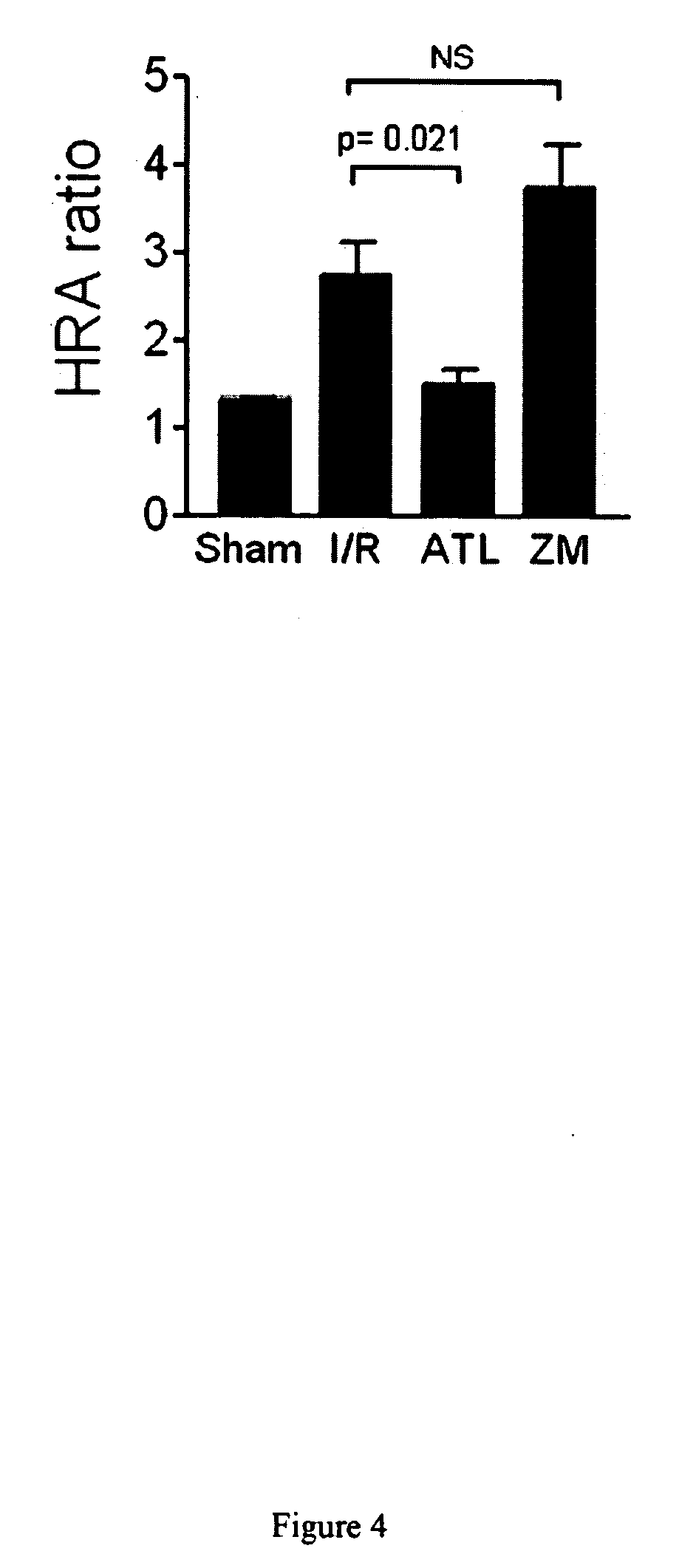
[0282]A mouse model has been enabled in which selective temporary occlusion of blood flow into the left liver lobe results in a profound acceleration of tumor growth in the clamped liver lobes, but not in the unclamped liver lobes. An A2A receptor agonist was then studied to determine its effect on the accelerated tumor growth.
[0283]Detailed Protocol
1. Before the Experiment: Animals
[0284]Male Balb / C mice (10-12 weeks) are housed under standard laboratory conditions with free access to water and chow and a 12-hour dark-light cycle.
2. Before the Experiment: Cell Culture
[0285]The murine colon carcinoma cell line C26 is cultured in Dulbecco's modified Eagle's medium supplemented with 5% heat-inactivated fetal calf serum, penicillin (100 units / ml) and streptomycin (100 μg / ml) in a 5% carbon dioxide environment.
3. Day 0: Induction of Liver Metastases
[0286]Confluent cultures of the C26 cell line are harvested by brief trypsinization (0.05 trypsin in 0.02% EDTA).[0287]After centrifugation, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com