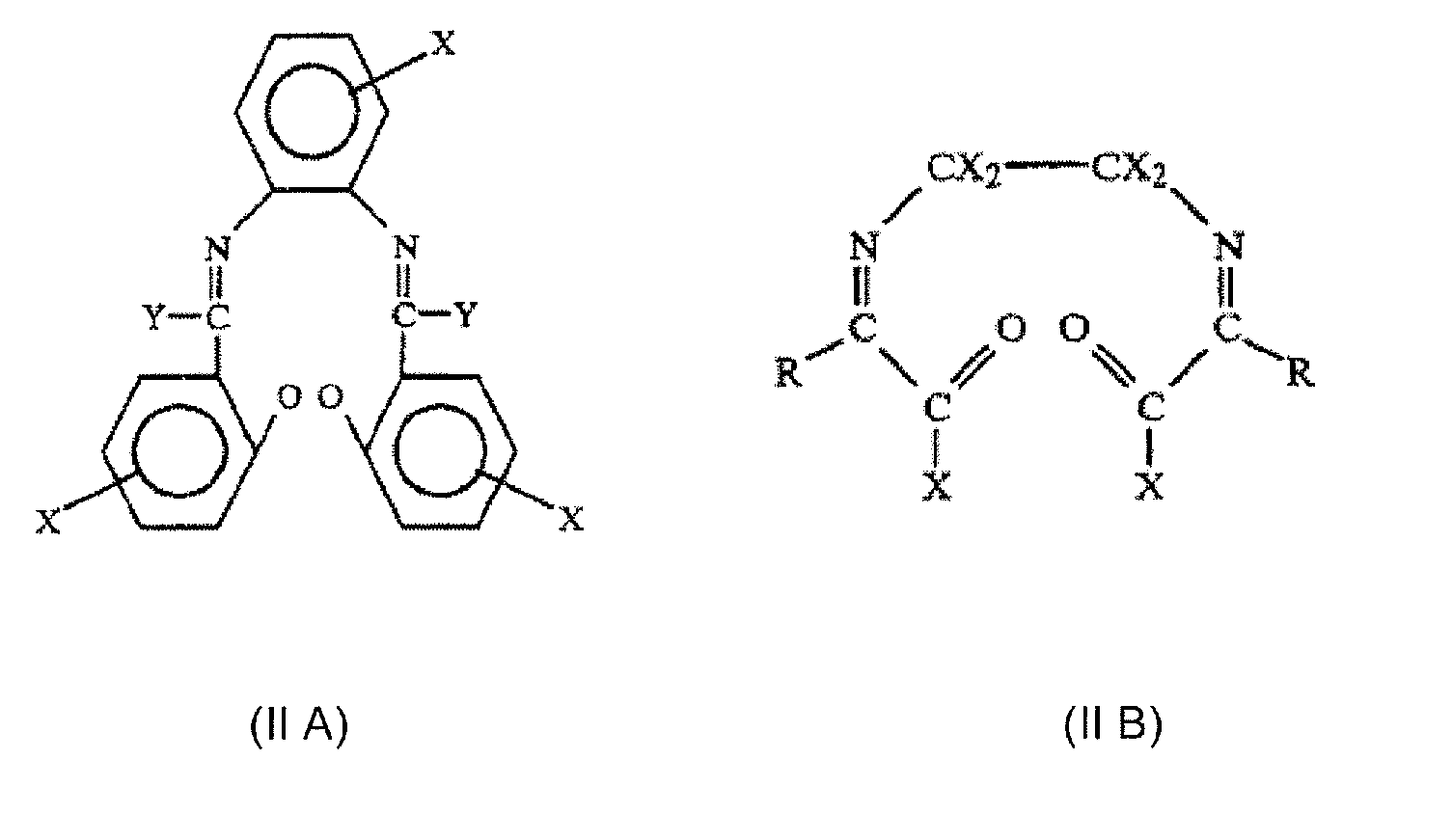
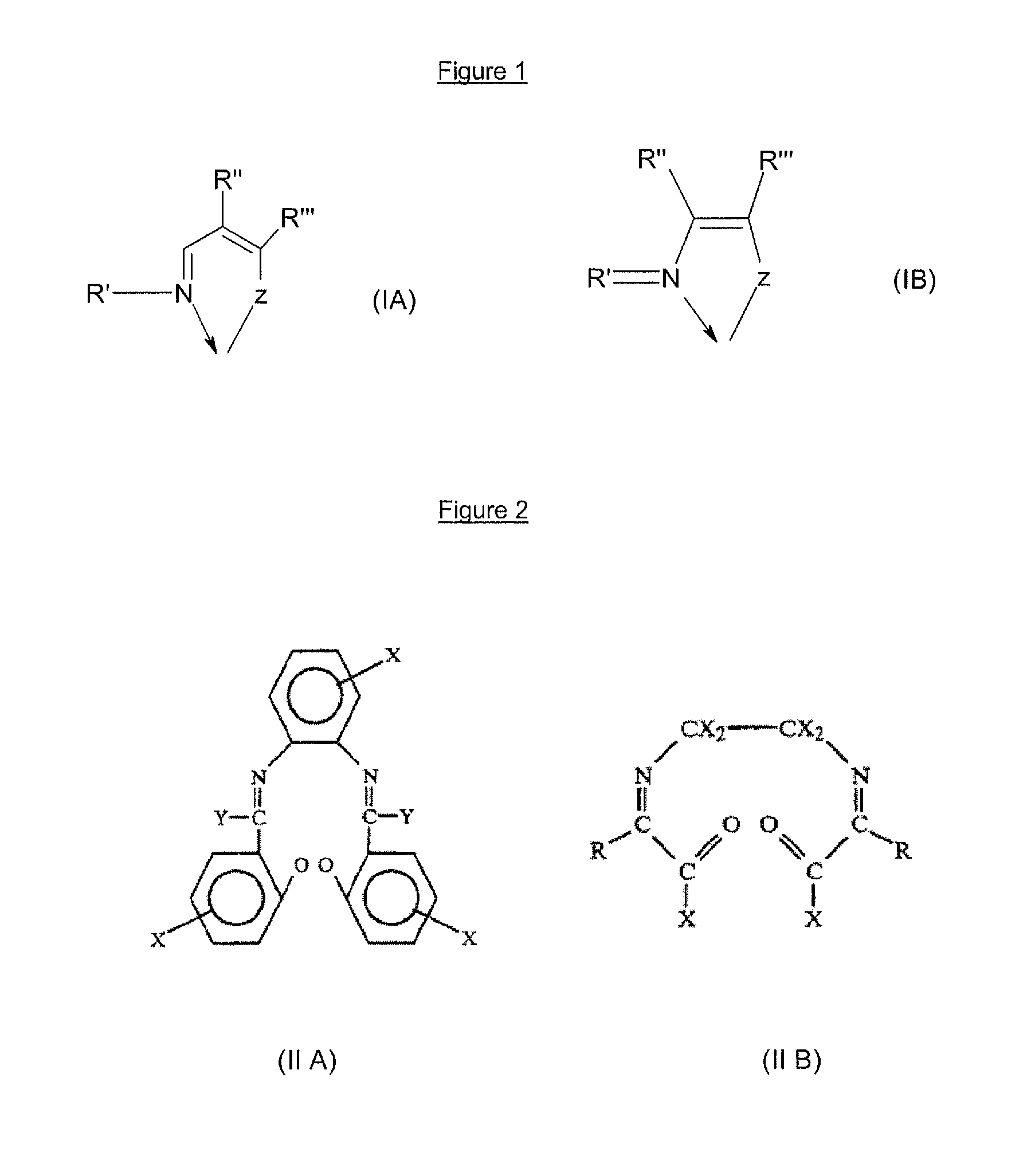
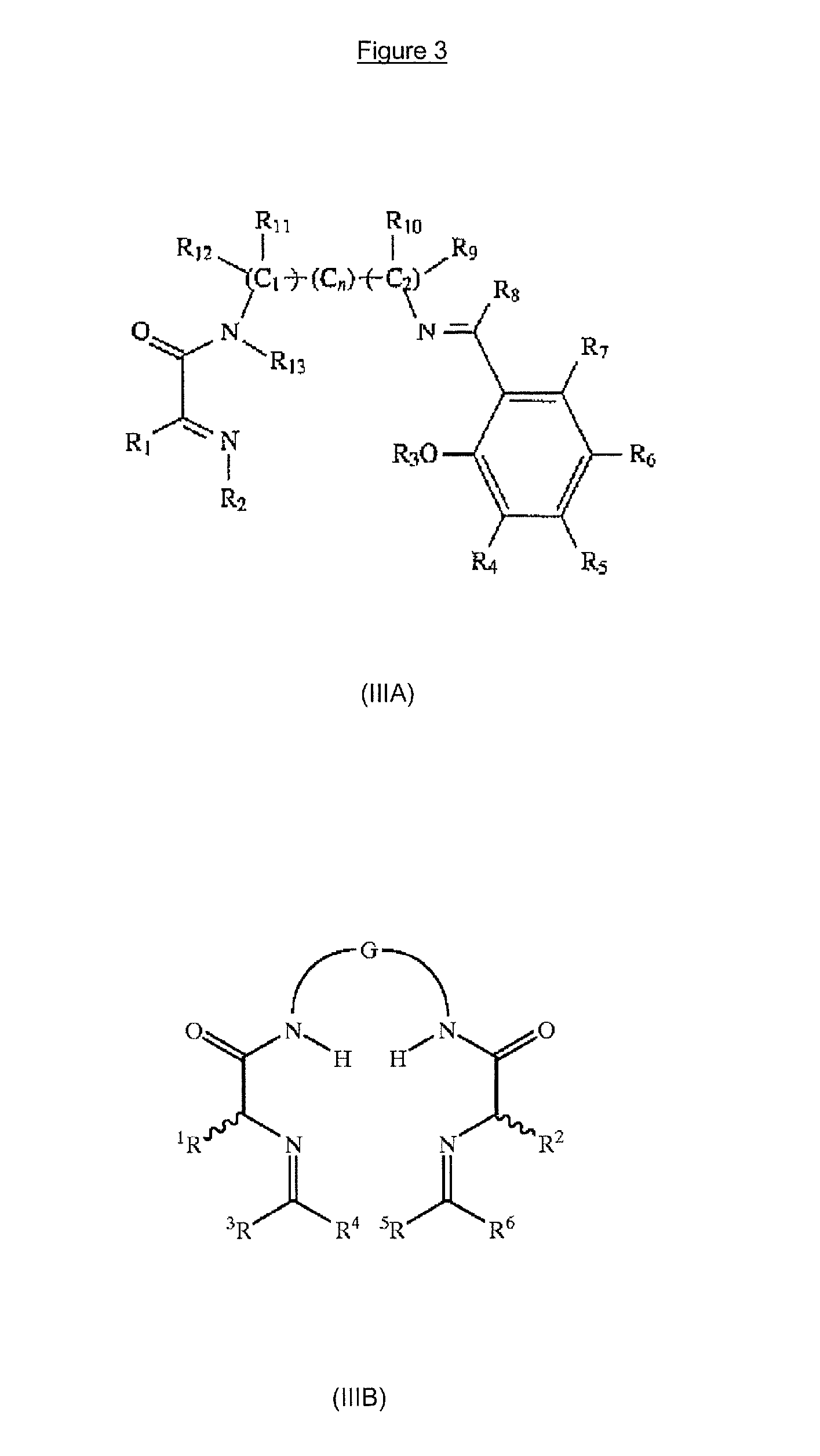
Multicoordinated metal complexes for use in metathesis reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-a to 1-e
Preparation and Characterisation of Schiff Base Ligands
[0335] The following Schiff base ligands were prepared, purified and characterised as disclosed in WO 2005 / 035121: [0336] N-(2,6-diisopropylphenyl)-2-hydroxy-3-tertbutyl-1-phenylmethaneimine (Schiff base 1-A) represented by the structural formula: [0337] N-(4-bromo-2,6-dimethyl)-2-hydroxy-3-tertbutyl-1-phenylmethaneimine (Schiff base 1-B) represented by the structural formula: [0338] N-(4-bromo-2,6-dimethylphenyl)-2-hydroxy-1-phenylmethaneimine (Schiff base 1-C) represented by the structural formula: [0339] N-(4-bromo-2,6-dimethylphenyl)-2-hydroxy-4-nitro-1-phenylmethaneimine (Schiff base 1-D) represented by the structural formula: [0340] N-(2,6-diisopropylphenyl)-2-hydroxy-4-nitro-1-phenylmethaneimine (Schiff base 1-E) represented by the structural formula:
examples 2 to 8
Preparation and Characterisation of Schiff Base Substituted Ruthenium Complexes
[0341] The following ruthenium complexes coordinated with Schiff bases from examples 1-A to 1-E were prepared and characterised according to the procedure described in examples 2-8 of WO 2005 / 035121: [0342] example 2 (obtained from Schiff base 1-C and methyllithium) represented by the structural formula: [0343] example 3 (obtained from Schiff base 1-E and methyllithium) represented by the structural formula: [0344] example 4 (obtained from Schiff base 1-B and methyllithium) represented by the structural formula: [0345] example 5 (obtained from Schiff base 1-A and phenylmagnesium chloride) represented by the structural formula: [0346] example 6 (obtained from Schiff base 1-A in the second step) represented by the structural formula: [0347] example 7 (obtained from Schiff base 1-A and methyllithium) represented by the structural formula: [0348] example 8 (obtained from Schiff base 1-A and pentafluoro...
examples 9 and 10
Preparation and Characterisation of Bimetallic Schiff Base Substituted Ruthenium Complexes
[0349] The two following bimetallic Schiff base substituted ruthenium complexes were made according to the procedure described in WO 2005 / 035121 (examples 9-10): [0350] example 9 represented by the structural formula: [0351] example 10 represented by the structural formula:
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact time | aaaaa | aaaaa |
| contact temperature | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com