Salts of benzimidazole derivative with amines and process for manufacturing the same
a technology of benzimidazole and amines, which is applied in the field of salts of benzimidazole derivatives with amines and process for manufacturing the same, can solve the problems of ineffective crystallization process of salts of compounds such as omeprazole that act as proton pump inhibitors with amines, and no method of obtaining alkali metal salts, etc., and achieves efficient removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
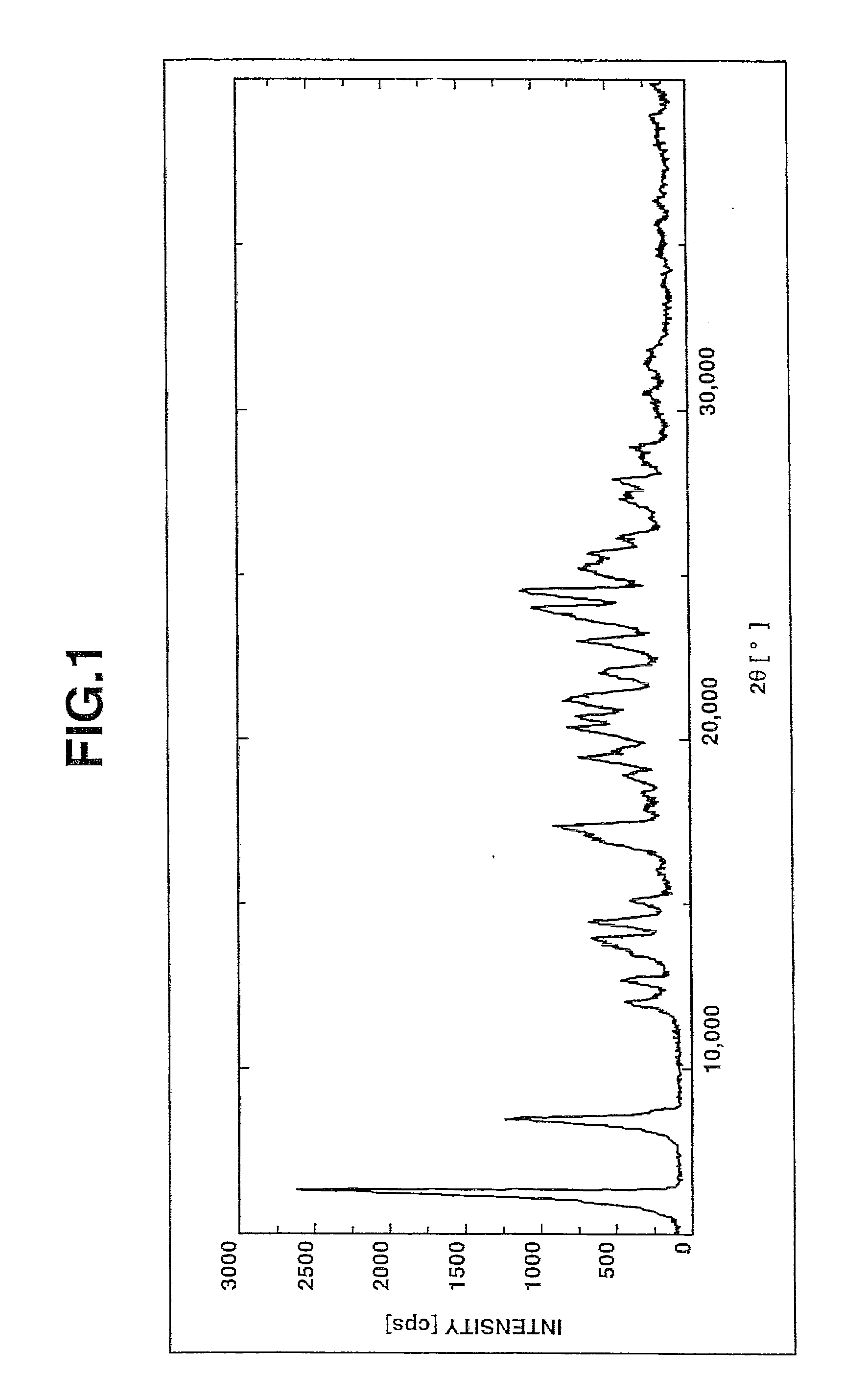
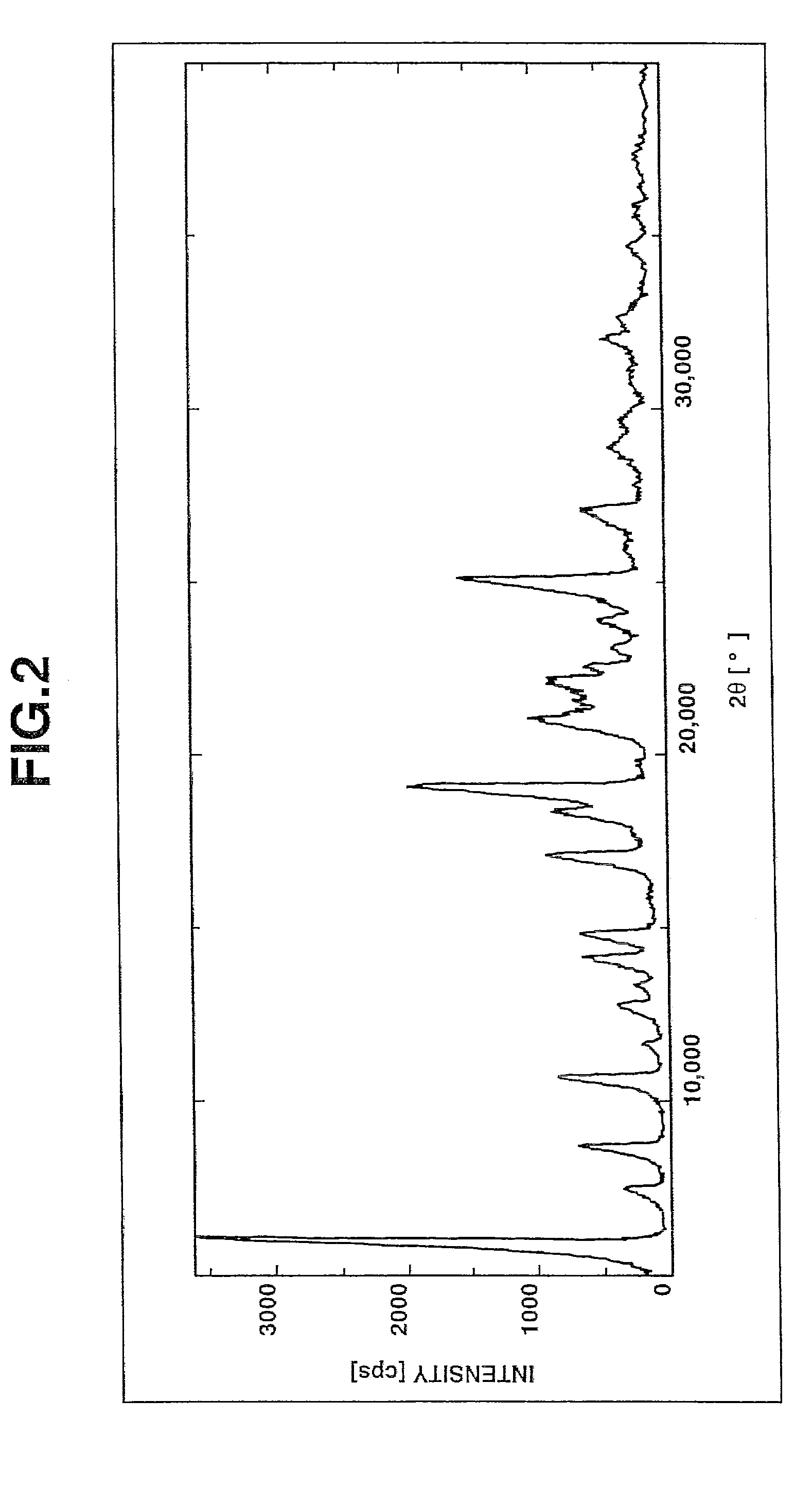
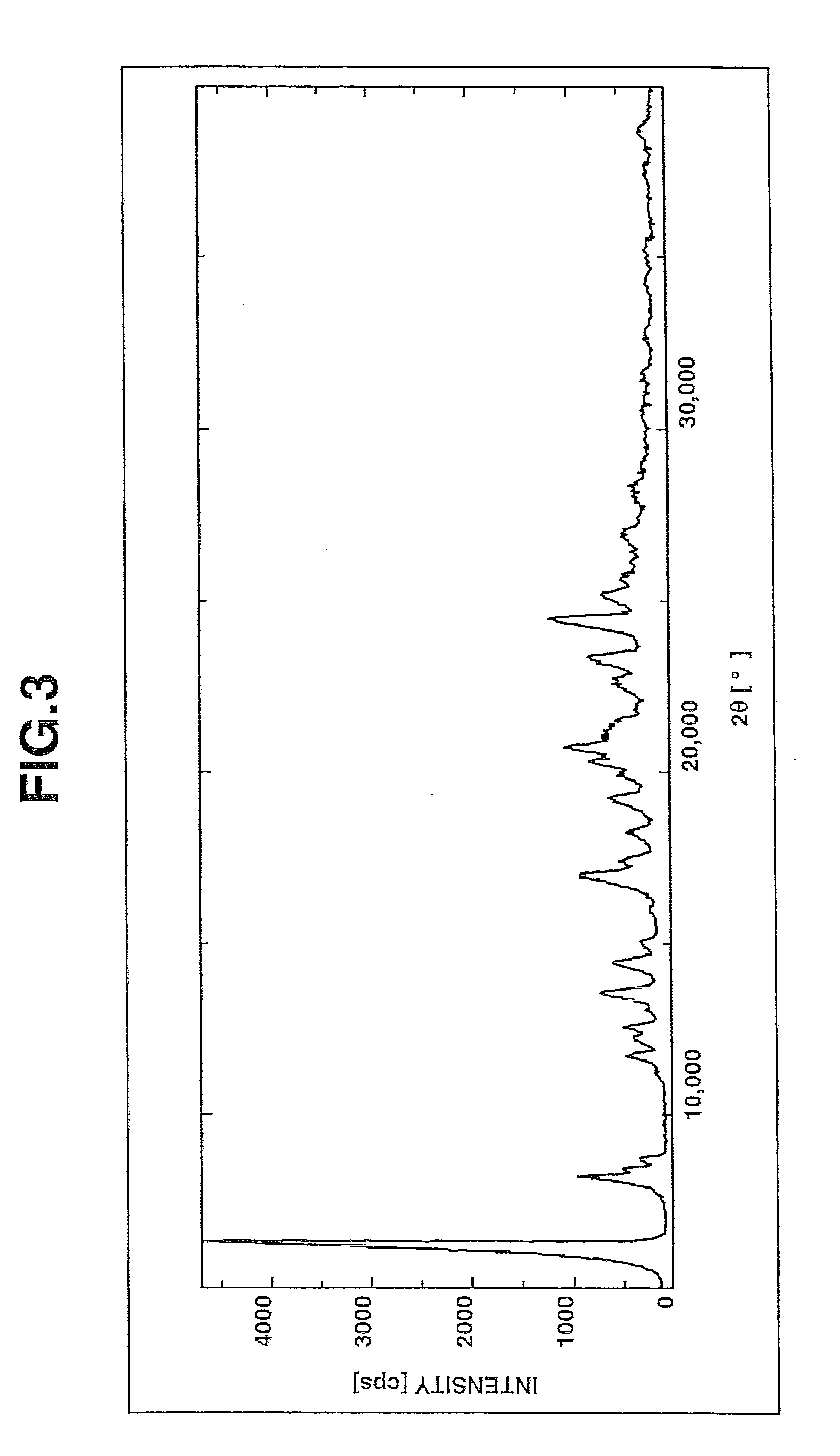
Image
Examples
reference example 1
[0097]2-Hydroxymethyl-4-(3-methoxypropoxy)-3-methylpyridine (5.0 g (23.7 mmol)) was dissolved in toluene (40 ml), and thionyl chloride (4.23 g (35.6 mmol)) was added dropwise thereto so that the temperature did not exceed 25° C. Following stirring at room temperature, disappearance of the raw materials was confirmed by TLC, and 2-chloromethyl-4-(3-methoxypropoxy)-3-methylpyridine (6.13 g) was obtained by concentrating under a reduced pressure (yield: 97.3%).
reference example 2
[0098]2-Hydroxymethyl-4-(3-methoxypropoxy)-3-methylpyridine (5.0 g (23.7 mmol)) was dissolved in ethyl acetate (40 ml), and thionyl chloride (4.23 g (35.6 mmol)) was added dropwise thereto so that the temperature did not exceed 25° C. Following stirring at room temperature, disappearance of the raw materials was confirmed by TLC, and 2-chloromethyl-4-(3-methoxypropoxy)-3-methylpyridine (6.14 g) was obtained by concentrating under a reduced pressure (yield: 97.4%).
reference example 3
[0099]2-Hydroxymethyl-4-(3-methoxypropoxy)-3-methylpyridine (5.0 g (23.7 mmol)) was dissolved in dimethoxyethane (40 ml), and thionyl chloride (4.23 g (35.6 mmol)) was added dropwise thereto so that the temperature did not exceed 25° C. Following stirring at room temperature, disappearance of the raw materials was confirmed by TLC, and 2-chloromethyl-4-(3-methoxypropoxy)-3-methylpyridine (6.25 g) was obtained by concentrating under a reduced pressure (yield: 99.2%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| stirring time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com