Antisense Oligonucleotides Against VR1
a technology of anti-vr1 and oligonucleotides, which is applied in the field of anti-vr1 anti-oligodeoxynucleotides, can solve the problems of increasing the number of side effects of existing analgesics, and increasing the number of receptors per cell. , to achieve the effect of preventing the development of hyperalgesia and allodynia and reducing the number of receptors per cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Generally Suitable Restriction Sites
[0106]The first step in the antisense and ribozyme strategy is to identify accessible sites on the mRNA for binding oligonucleotides, in particular ribozymes. To this end, the VR1 mRNA had to be investigated for such restriction sites. Analysis of the VR1 mRNA revealed the following potential recognition sites for ribozymes in the coding domain:
[0107]33×GT(U)C sequences,
[0108]28×GT(U)T sequences, and
[0109]12×GT(U)A sequences.
[0110]In order to determine the accessible sites on the VR1 mRNA, in a first step, three independent nucleotide mixtures with the following sequence were synthesized:[0111]Mixture 1: NNNAACNNN “GUU library,”[0112]Mixture 2: NNNCACNNN “GUA library,” and[0113]Mixture 3: NNNGACNNN “GUC library.”
[0114]These were used consecutively in an RNase H experiment and it was found that appreciable degradation of the VR1 mRNA was observed only with the GUC library. Thus, of the potential target sequences for ribozymes, the...
example 2
Identification of the Most Effective Antisense Oligodeoxynucleotides
[0115]Messenger Walk Screening
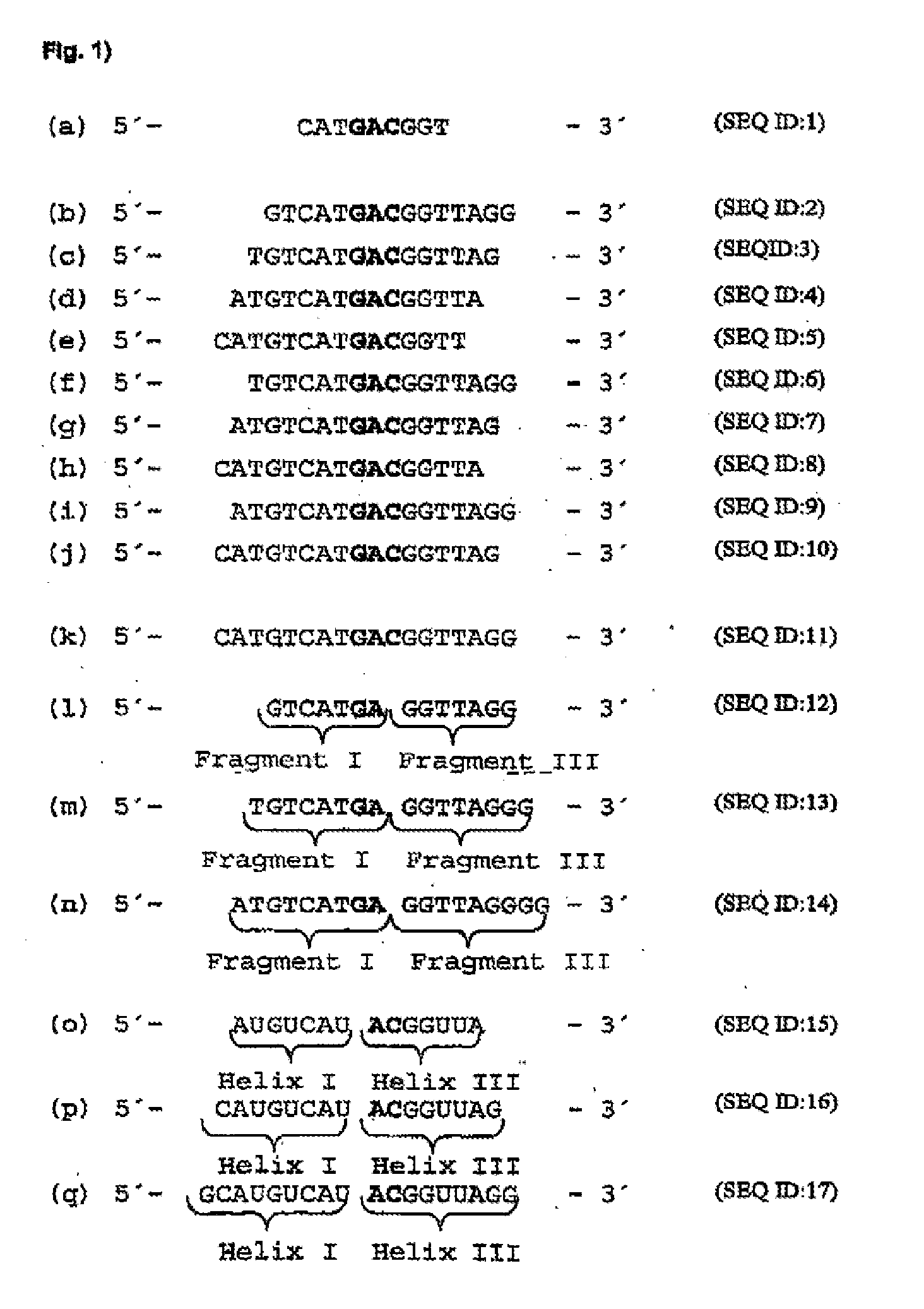
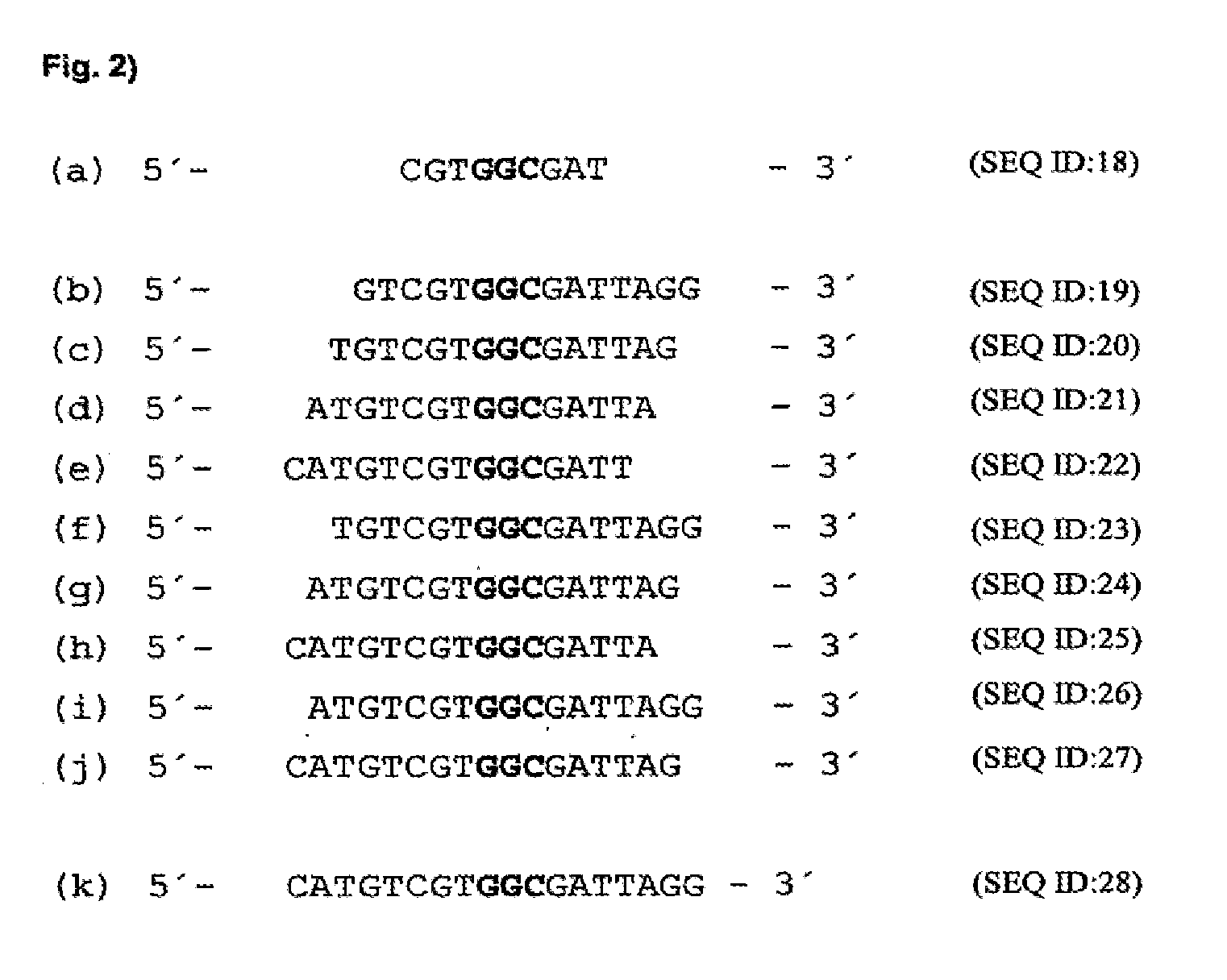
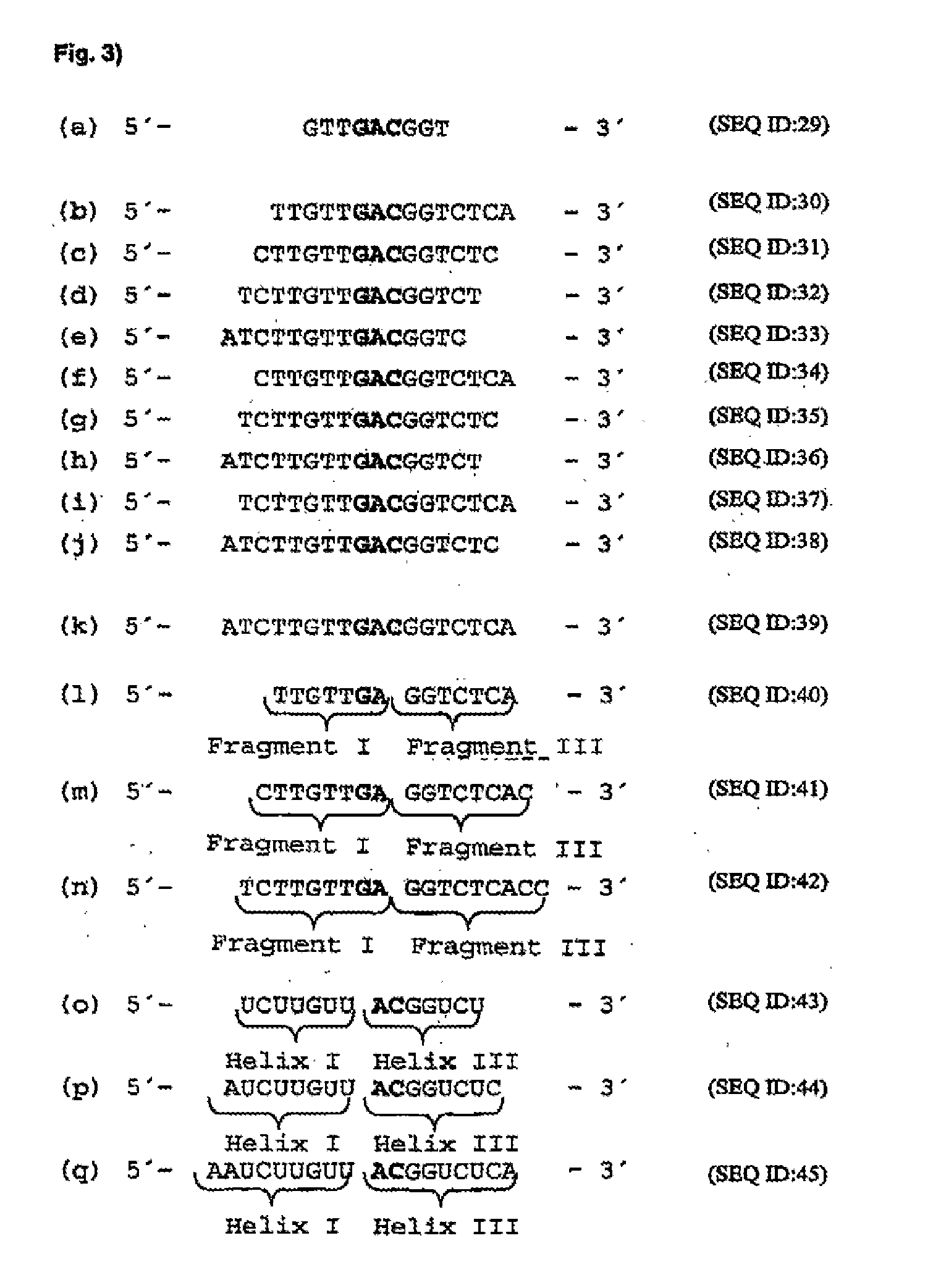
[0116]In order to identify mRNA domains which are accessible to antisense oligodeoxynucleotides, the mRNA was systematically screened with ODNs in an RNase H assay (messenger walk screening). The ODNs were 18 nucleotides in length and contained a central GAC sequence, which is reverse complementary to GUC sequences in the mRNA. This triplet was selected as the target as it provided good results and may be used in a second step to develop hammerhead ribozymes and DNA enzymes. In total, 33 ODNs, designated V1 to V33, were tested against all the GUC sites of the VR1 mRNA. The ODNs were systematically screened for their suitability by the addition in each case of one ODN and RNase H to the mRNA. RNase H cuts formed DNA / RNA duplexes wherever an oligonucleotide can bind to the mRNA (FIG. 17).
[0117]In Vitro Transcription of VR1 mRNA
[0118]First of all, the cDNA of the vanilloid receptor was clo...
example 3
Ribozymes and DNA Enzymes
[0129]On the basis of the identified most effective binding sites, corresponding ribozymes and DNA enzymes were also investigated.
[0130]“Hammerhead” ribozymes and type “10-23” DNA enzymes (Santoro et al., 1997) were constructed against the mRNA sites which were accessible to ODNs. The length of the “recognition arms” was 7 or 9 nucleotides on each side. Quantitative evaluation of mRNA cleavage / restriction under “single turnover” conditions (10-fold excess of ribozymes and DNA enzymes) revealed after 20 minutes at 37° C. that ribozymes with shorter “recognition arms” (helix I and helix III) are more active, while in turn in DNA enzymes, those with longer “arms” (Fragment I and Fragment III) are more active (FIG. 20). FIG. 24 is a schematic representation of a ribozyme with the helices I and III, while FIG. 25 shows a specific example. This may be seen for a 10-23 DNA enzyme (5′-end (Fragment I) and 3′-end (Fragment III) from the catalytic motif) in Santoro et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com