Multi-component antioxidant compounds, pharmaceutical compositions containing same and their use for reducing or preventing oxidative stress
a technology of antioxidant compounds and pharmaceutical compositions, which is applied in the direction of dipeptides, peptide/protein ingredients, depsipeptides, etc., can solve the problems of unacceptably risky administration routes, cumbersome and invasive, ineffective reduction,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
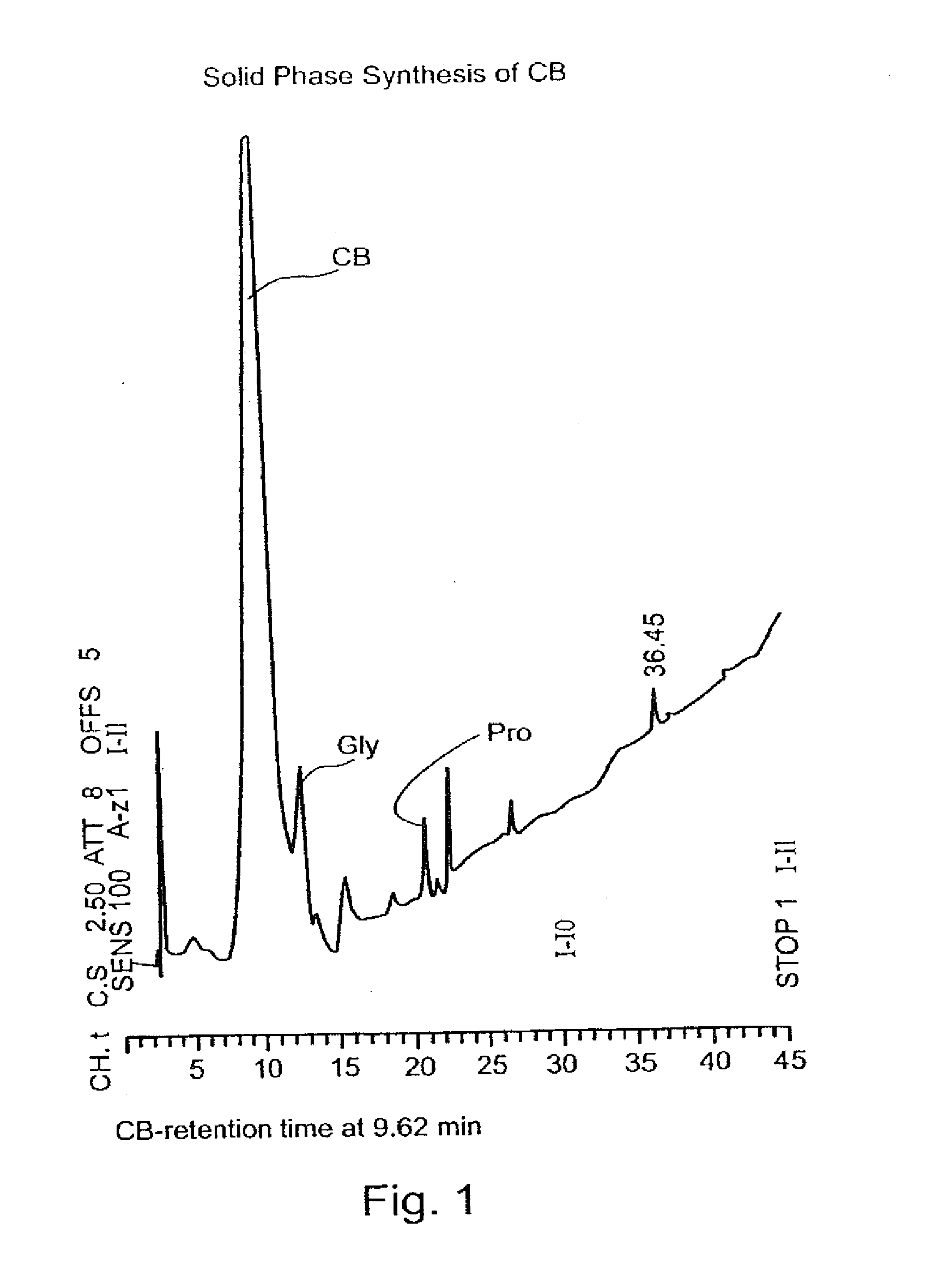
Synthesis of N-Acetyl Cysteine-Glycine-Proline-Cysteine-Amid
[0135]The synthesis of N-Acetyl Cysteine-Glycine-Proline-Cysteine-Amid (CB, SEQ ID NO:1) having the chemical structure of:
CH3CO—NH—CH(CH2SH)CO—NHCH2CO—N(CH2—CH2—CH2)CH—CO—NH—CH(CH2SH)—CO—NH2
(molecular weight of 406) is described herein.
[0136]Synthesis: CB was prepared by solid phase synthesis of peptides according to published protocols. The synthesis was carried out according to Fastmoc 0.25 mmol modules in a peptide synthesizer Model 433A (Applied Biosystems) according to the User's manual.
[0137]In particular, 9-fluorenylmethoxycarbonyl (Fmoc) amino acid (1 mmol) was dissolved and activated in the cartridge in a mixture of 3.0 g of 0.45 M 2-(1H-benzoltriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU / HOBt) in DMF, 2 M Diisopropyethylamine (DIEA) and 0.8 ml N-methyl-pyrrolidone (NMP). De-protection was carried out in 22% piperidine solution in NMP. All steps were carried out under nitrogen.
[0138]De protect...
example 2
Inhibition of JNK (c-Jun NH2-terminal kinase) and p38 enzymes
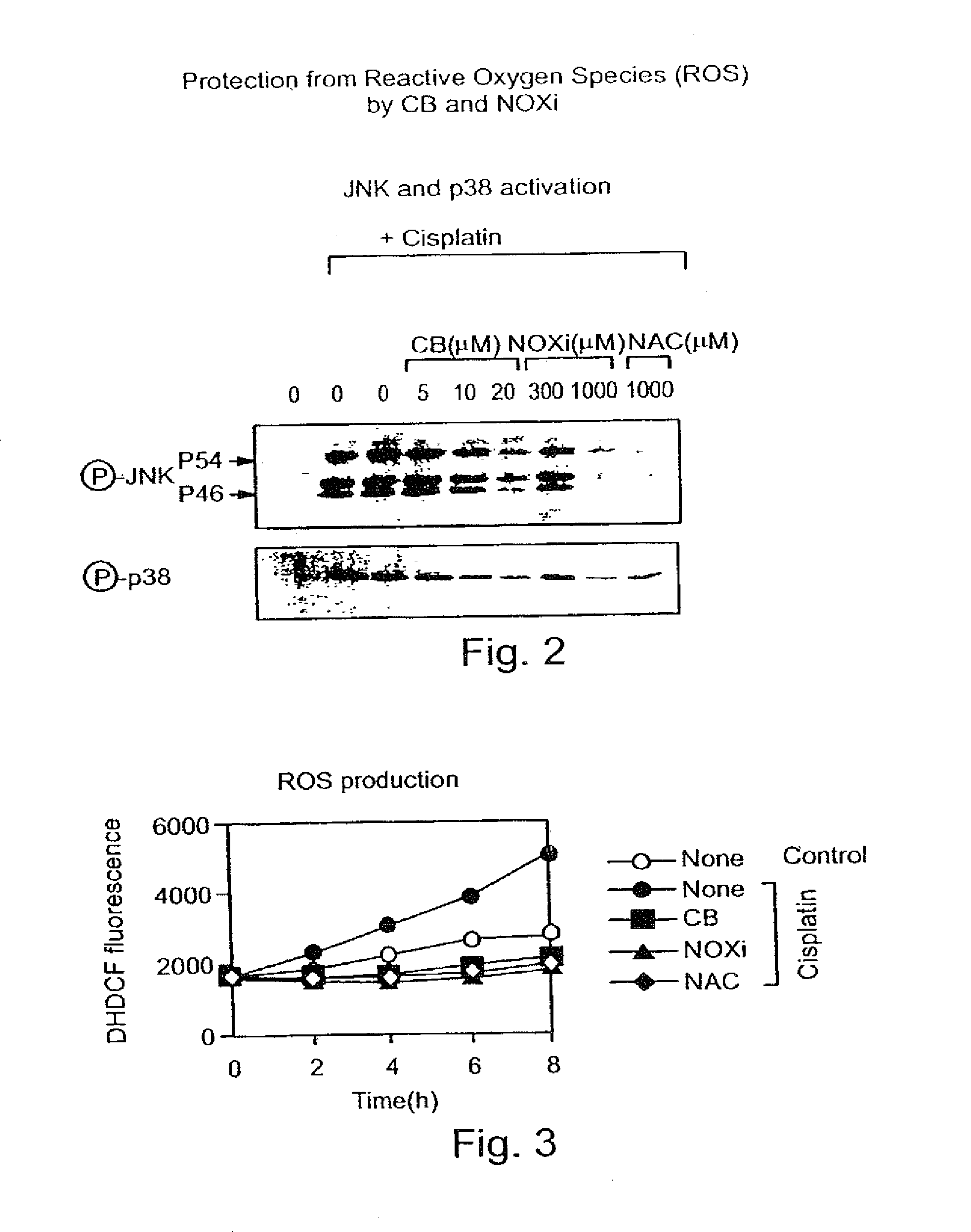
[0146]In order to show the efficacy of CB against a stimulant that activates oxidative stress, an inhibition assay of both JNK (c-Jun NH2-terminal kinase) and p38 enzymes in tissue culture was performed.
[0147]NIH3T3 cells overexpressing EGF receptor (DHER14 cells) (55) were exposed to cisplatin (CDDP, 30 μM) which activates specific enzymes involved in apoptosis including JNK and p38.
[0148]As shown in FIG. 2, JNK or p38 were detected by specific antibodies essentially as previously described (6). In the presence of increasing concentrations of CB, a dramatic reduction in the phosphrylated form of either p38 or JNK enzymes was obtained. In the presence of 20 μM CB, phosphorylated p38 and JNK enzymes were not detected at all. Two known antioxidants were used as positive controls, NOXi (at 300 and 1000 μM) and NAC (NAC) (at 1000 μM). The efficacy of CB at 20 μM was similar to that obtained by the addition of 1 mM of N-acetyl ...
example 3
Inhibition of ROS Production
[0149]The concentration of reactive oxygen species (ROS) in DHER14 cells following administration of antioxidants was determined using the ROS sensitive fluorescent dye DHDCF (fluoresceine derivative). As shown in FIG. 3, reduction of ROS below normal levels was prominent in the presence of 20 μM of CB. Two known antioxidants were used as control, NOXi (at 1000 μM) and NAC (NAC) (at 1000 μM). The efficacy of CB in reducing ROS was about ˜50 fold better then these two known antioxidants. Thus, at 20 μM CB was as efficient as 1000 μM NAC or 1000 μM NOXi.
PUM
| Property | Measurement | Unit |
|---|---|---|
| antioxidant | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
| miscibility properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com