Remotely Activated Piezoelectric Pump for Delivery of Biological Agents to the Intervertebral Disc and Spine
a piezoelectric pump and biological agent technology, applied in the field of implantable drug delivery systems, can solve the problems of over-high societal cost, most lost productivity, and the largest amount of total lost time, and achieve the effect of negligible difference in the acceleration of the two vertebra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
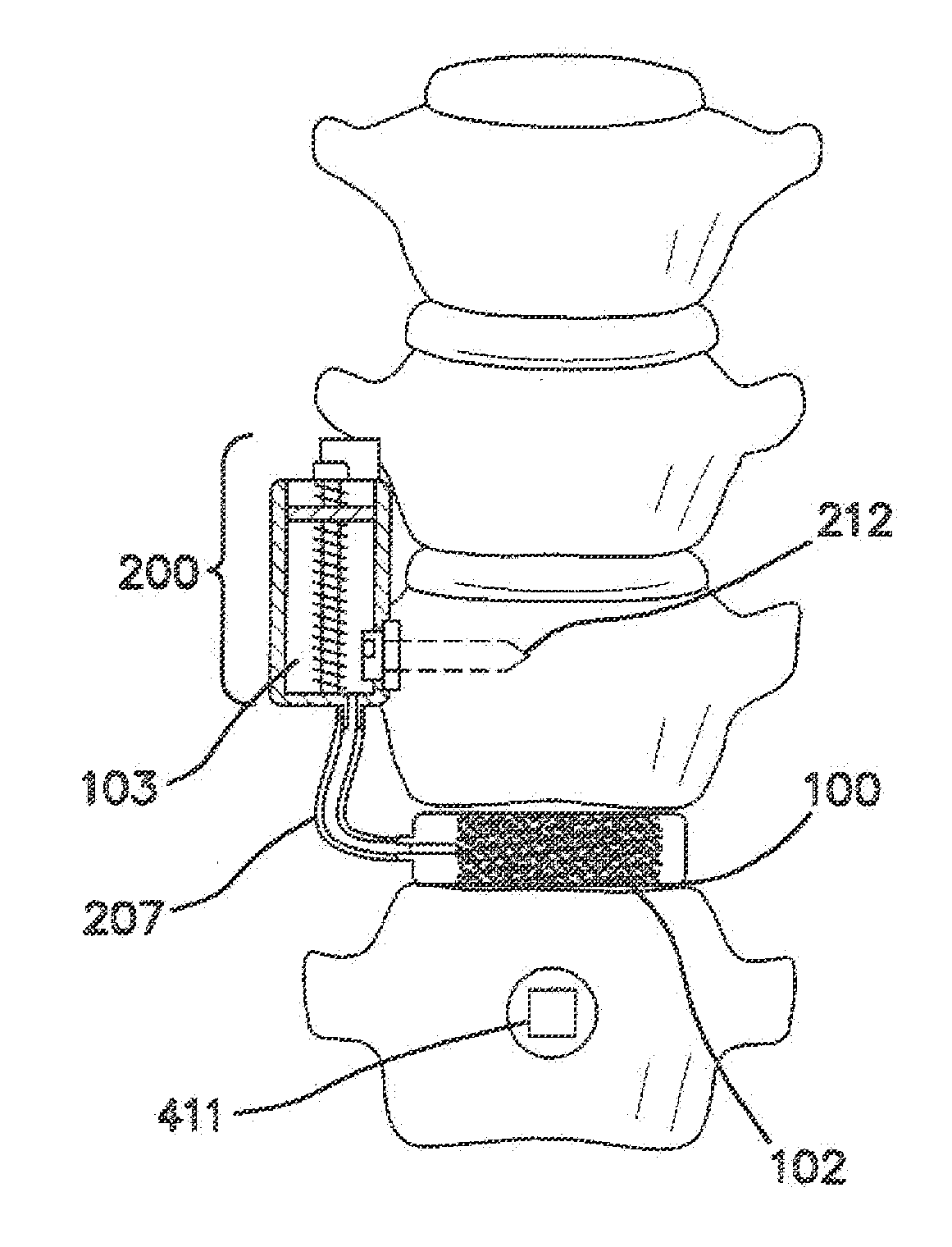
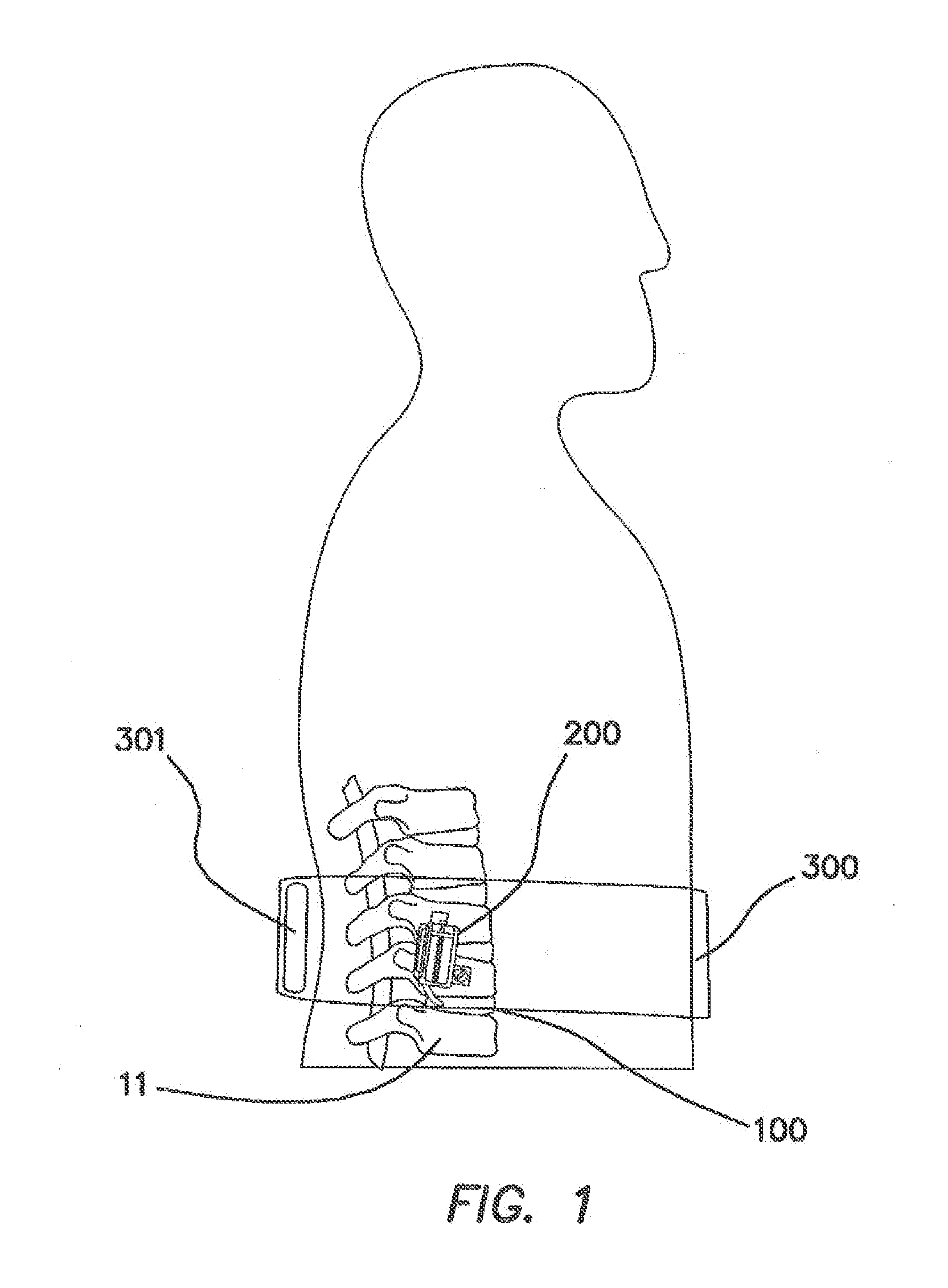
[0062]FIG. 1 is a lateral cross sectional view of the disclosed spinal implant system in its relation to the spine of a patient. The system is intended for lumbar spinal vertebrae 11 located in the low back side of the human body. The spinal implant system comprises an implanted spinal pump 200 which delivers biological agents such as BMP-2 to an interbody spinal cage 100 that is substantially doughnut shaped. The spinal implant system also comprises an external induction unit 300 that charges and controls the spinal pump 200 and via an inductive link. The induction unit 300 comprises an inductive charger and control unit 301.
[0063]FIGS. 2a and 2c show the front (anterior) and lateral side view respectively of the lumbar spine 10, spinal nerve 15, and normal vertebrae discs 12 before the replacement of an interbody disc 13 with the interbody spinal cage 100. FIGS. 2b and 2d show the anterior and lateral side view respectively of the lumbar spine 10, spinal nerve 15, and normal verte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com