Compositions and methods of treating pulmonary hypertension
a technology of pulmonary hypertension and compositions, applied in the direction of drug compositions, elcosanoid active ingredients, extracellular fluid disorders, etc., can solve the problems of difficult oxygenation of patients with pulmonary hypertension, difficult for the heart to pump blood through the lungs to be oxygenated, and high pulmonary arterial pressur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
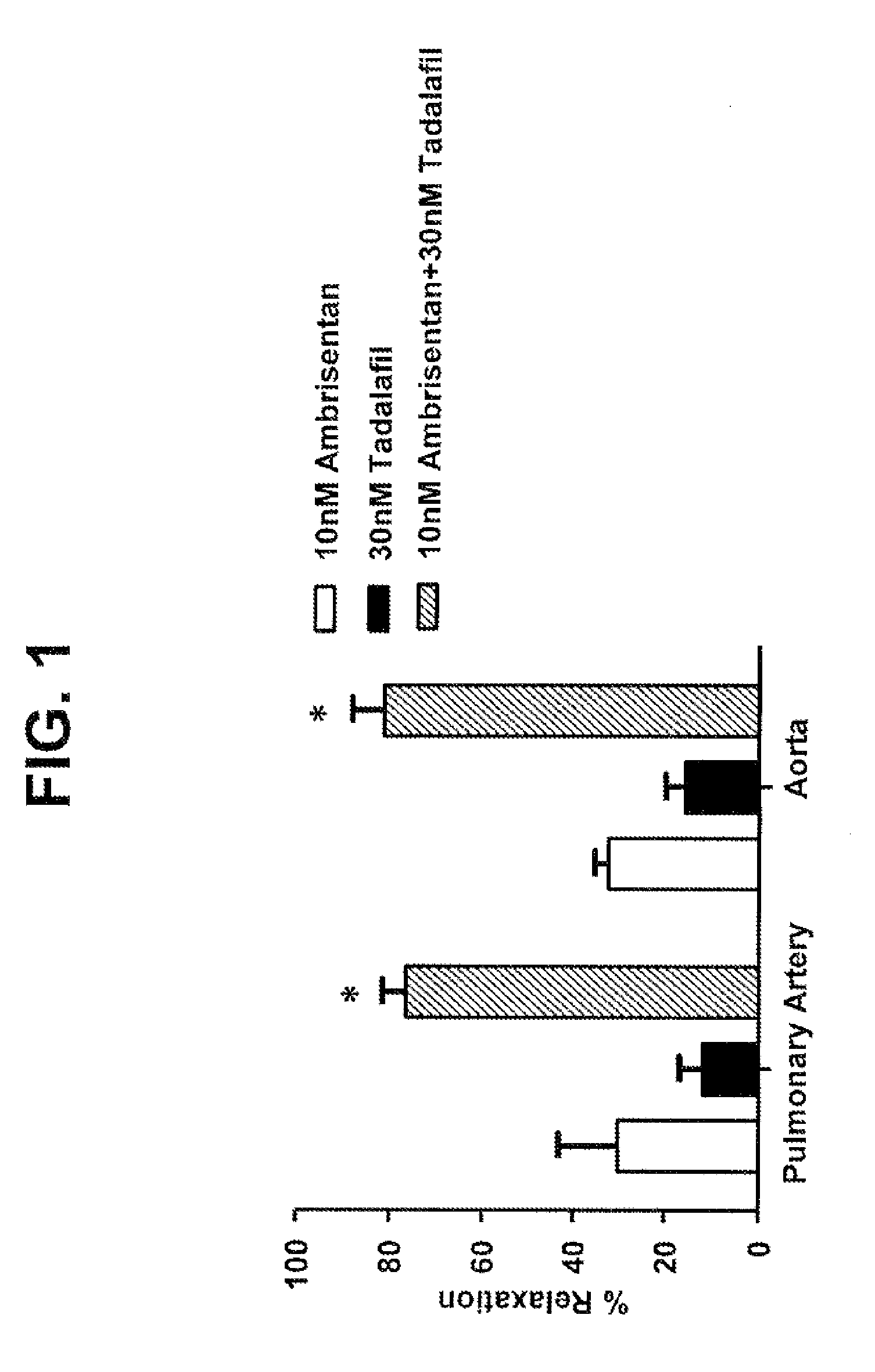
[0228]Ambrisentan and Tadalafil Relax Endothelin-Induced Contraction of Rat Pulmonary Arteries and Aortas
[0229]This example examines the pharmacological effects of the combination of ambrisentan and tadalafil in comparison with either of them alone, with respect to their capability to relax isolated rat pulmonary artery and thoracic aorta preparations.
[0230]The selective type-A endothelin receptor antagonist, ambrisentan (Letairis®), and the phosphodiesterase type 5 inhibitor, tadalafil (Adcirca®), are currently used to treat pulmonary arterial hypertension. Isolated rat intact intrapulmonary arterial rings contracted with 8 nM endothelin-1 (ET-1) were relaxed by 10 nM ambrisentan (from Gilead Sciences, Inc.) and 30 nM tadalafil (from Sequoia Research Products Ltd, Pangbourne, UK) by 30±13% (mean±SEM, n=3) and 12±5% (n=3), respectively, whereas both drugs in combination relaxed the intact intrapulmonary arterial rings by 77±5% (FIG. 1, n=3, P<0.01 vs. mono-administration of ambrisen...
example 2
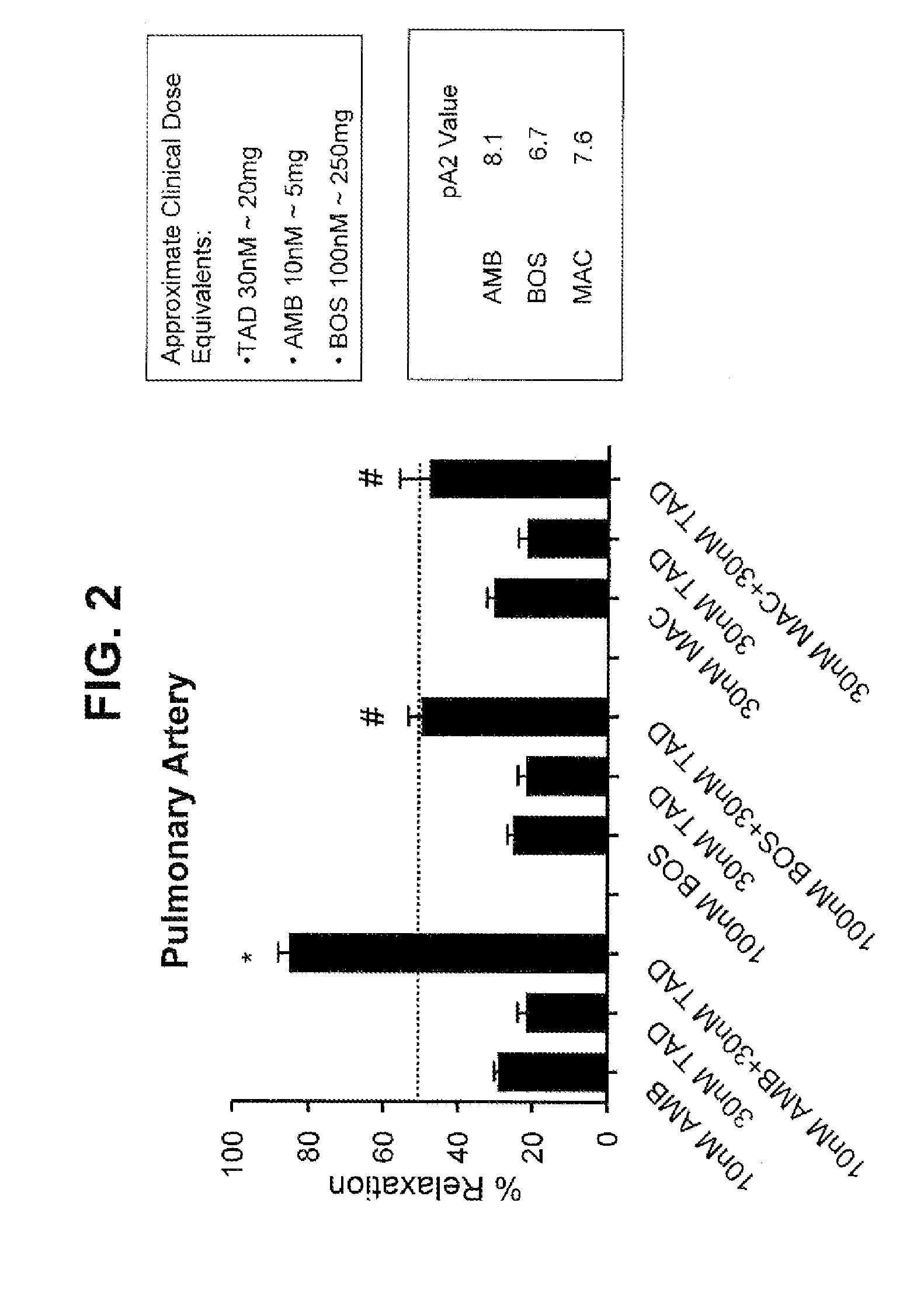
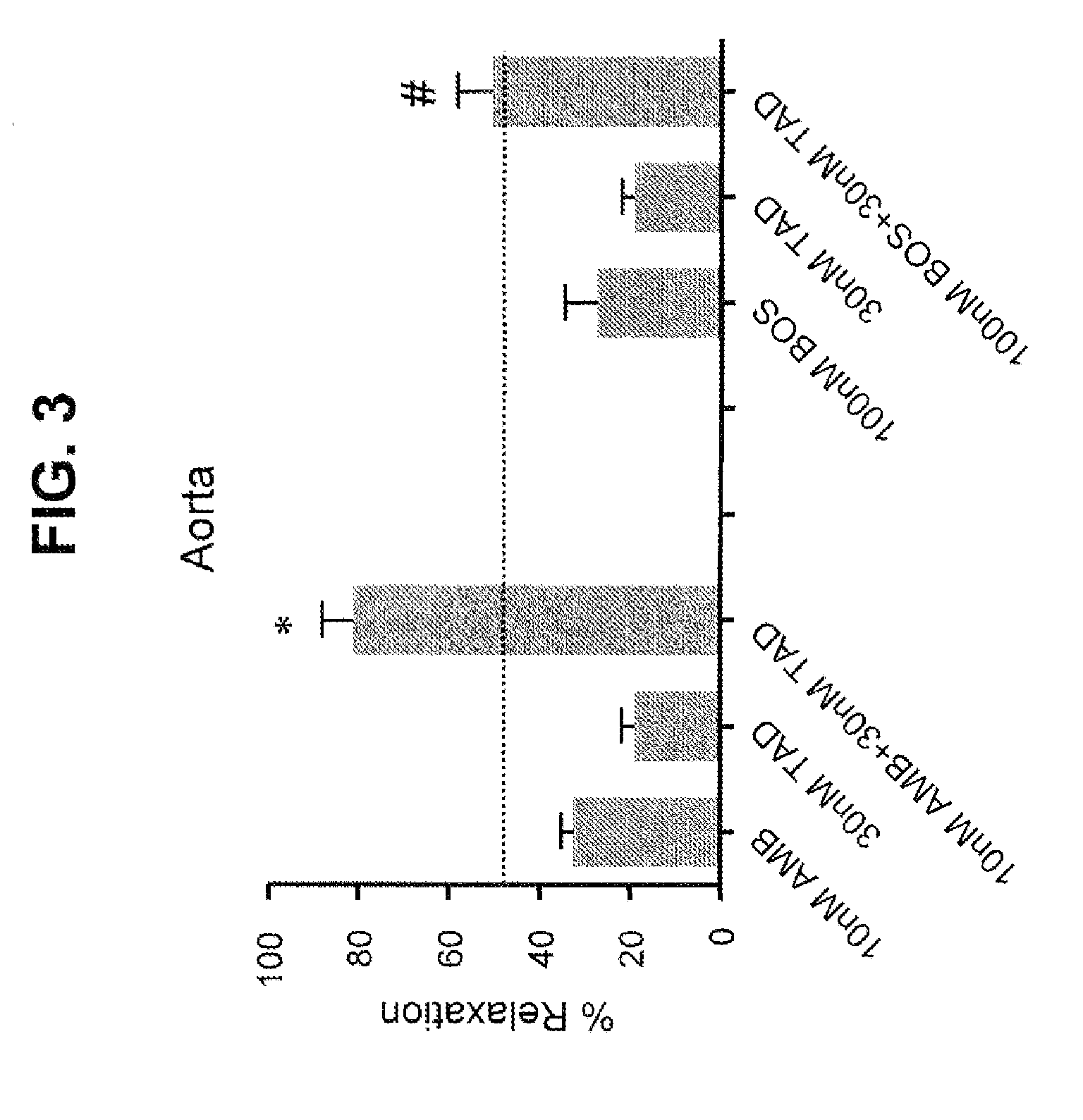
[0234]Selective Type-A ERA and PDE5 Inhibitor Shows Co-Action in Relaxing Endothelin-Induced Contraction of Pulmonary Arteries while Non-Selective ERA and PDE5 Inhibitor Lack Such Co-Action
[0235]This Example confirms the beneficial co-action of ambrisentan and tadalafil as, observed in Example 1 and further investigates the mechanism underlying such co-action.
[0236]Ambrisentan (Letairis®) is a selective type-A endothelin receptor antagonist approved for treatment of PAH. Bosentan (Tracleer®) is a non-selective (types A&B) endothelin receptor antagonist for PAH. Macitentan (second generation of Bosentan) is a non-selective endothelin receptor antagonist in phase III for PAH. Tadalafil (Adcirca® and Clalis®) is a PDE5 inhibitor for PAH and erectile dysfunction (ED).
Method: Ex-Vivo Vascular Function Assay
[0237]Intrapulmonary arteries (200-500 μm) and aortas were isolated from Sprague Dawley rats (300-320 g) and cut into 1-2 mm rings. Rings were mounted in a myograph and constricted wit...
example 3
[0247]Selective Type-A ERA and PDE5 Inhibitor Attenuated Hypoxia-Induced Pulmonary Arterial Hypertension (PAH)
[0248]This Example demonstrates the co-action of ambrisentan and tadalafil in a pulmonary arterial hypertension (PAH) animal model.
Method: Pulmonary Arterial Hypertension (PAH) Animal Model
[0249]Male SD Rats (225-250 g) were housed in chambers under normoxic (sea level) or hypoxic (10% oxygen) conditions for 3 weeks.
[0250]Rats were dosed with vehicle (0.5% hydroxypropyl methylcellulose (HPMC), 0.2% Tween 80, and 0.9% benzyl alcohol in water), AMB or TAD (quaque die) beginning the day they were placed in chambers. Plasma was collected when animals were terminated. Table 1 lists the animals used in this study, along with the treatments they received.
TABLE 1GroupNManipulationTreatmentDuration18NormoxiaVehicle3 wks212HypoxiaVehicle3 wks312Hypoxia10mpk TAD3 wks412Hypoxia1mpk AMB3 wks512Hypoxia10mpk AMB3 wks612Hypoxia 1 mpk AMB + 10 mpk TAD3 wks712Hypoxia10 mpk AMB + 10 mpk TAD3 w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com