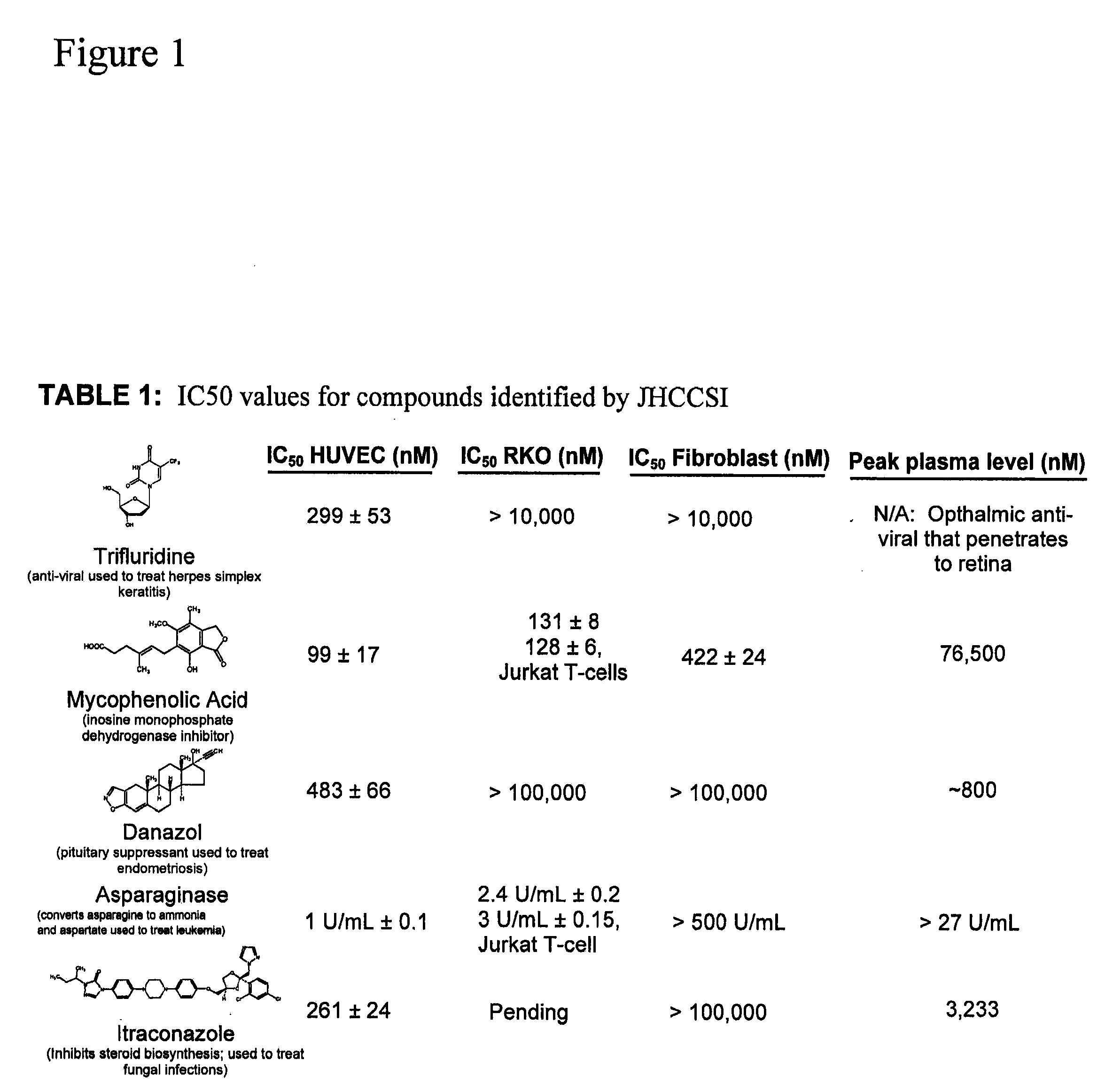
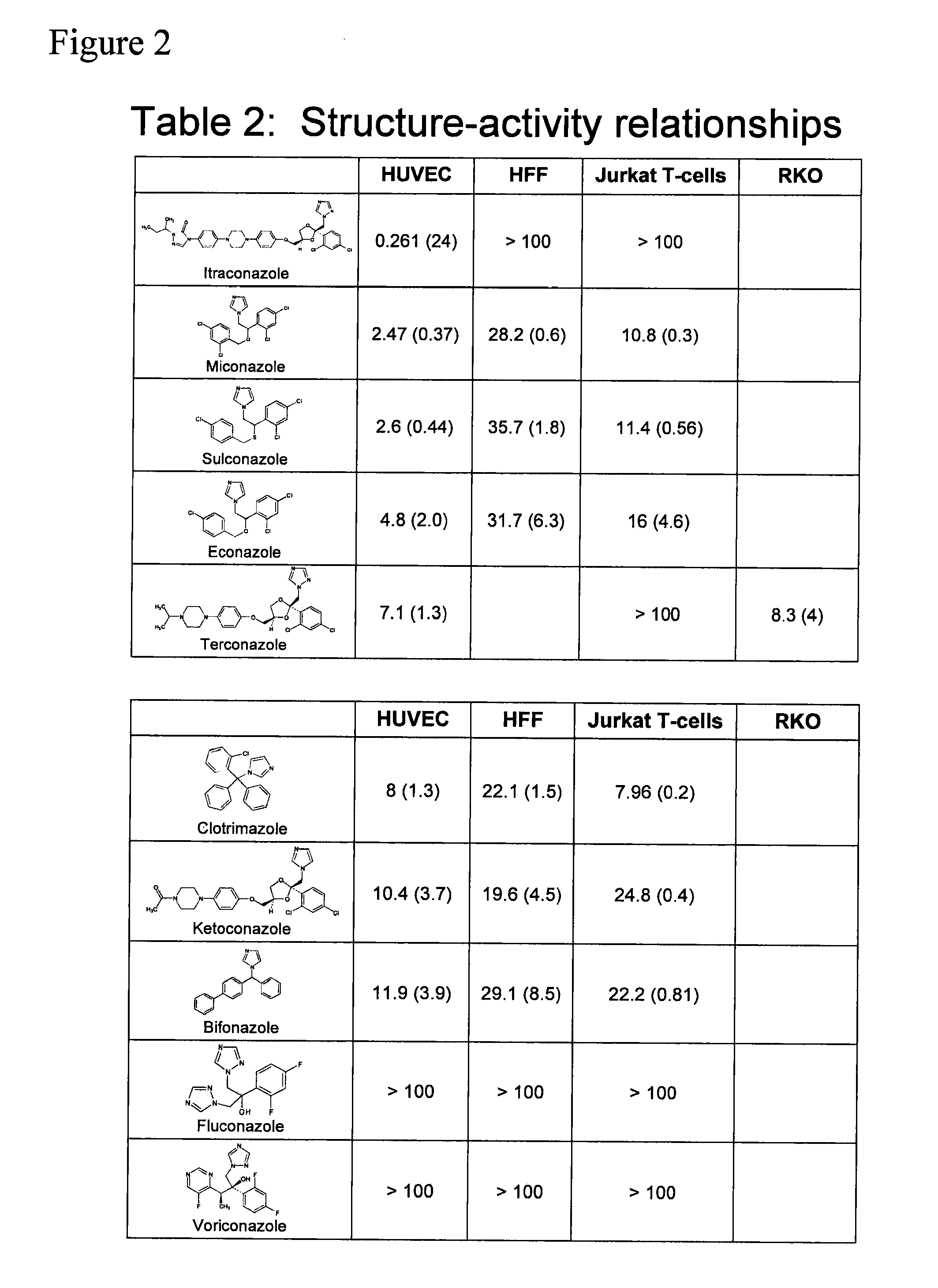
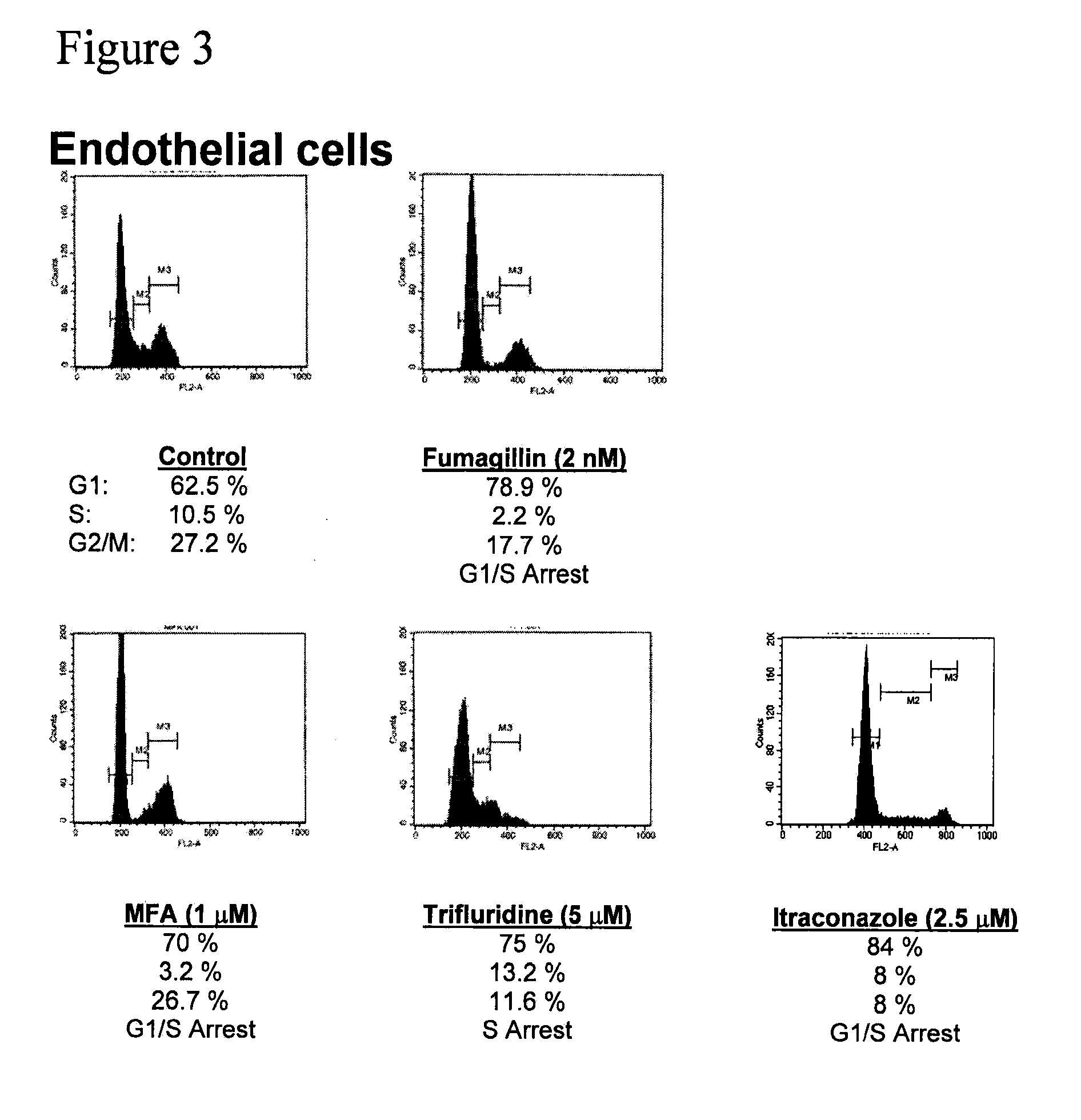
Angiogenesis inhibitors
angiogenesis inhibitor and inhibitor technology, applied in the field of angiogenesis inhibitors, can solve the problems of unstable, toxic, difficult to employ various materials, etc., and achieve the effect of removing or reducing normal but undesired tissue in a patien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials and Methods
[0241]Library construction.
[0242]32,000 FDA drug approvals obtained by Freedom of Information Act requests were condensed to 3,400 unique drug formulations. Drugs were purchased from Sigma, Spectrum Chemicals, MP Biomedicals, The Johns Hopkins Hospital pharmacy, Biomol, and Tocris. 10 mM stock solutions were made using DMSO, water, or ethanol as solvents. Drugs were arrayed in 96-well plates and screened at a final concentration of 10 μM. Cells were incubated with drug for 36 h and proliferation was measured by pulsing with 1 μCi [3H]-thymidine for 8 h followed by absorption onto glass filters and scintillation counting.
[0243]Statistical Analysis.
[0244]IC50 measurements were carried out in triplicate, and analyzed using four parameter logarithmic analysis. Values are presented + / − the standard error of the mean. P-values were calculated using the Student's T-test.
[0245]Semi-Quantitative RT-PCR.
[0246]RNA from subconfluent proliferating HUVEC (1×106) cells was pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| half life | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com